Latent heat is the heat required to transform a solid into a liquid or vapour phase. It is known by several names depending on its phase, such as the heat of condensation, the heat of vaporization, and so on. It can also refer to the quantity of heat energy received or emitted during a phase transition. We will look at the idea of latent heat and the latent heat formula with examples in this article.
What is Latent Heat?
The energy or heat released or absorbed during a phase change of a material is known as latent heat. It might be from a gas to a liquid or from a liquid to a solid and back again.
The graph given below shows, the change in various forms of matter and the heat related to each process.
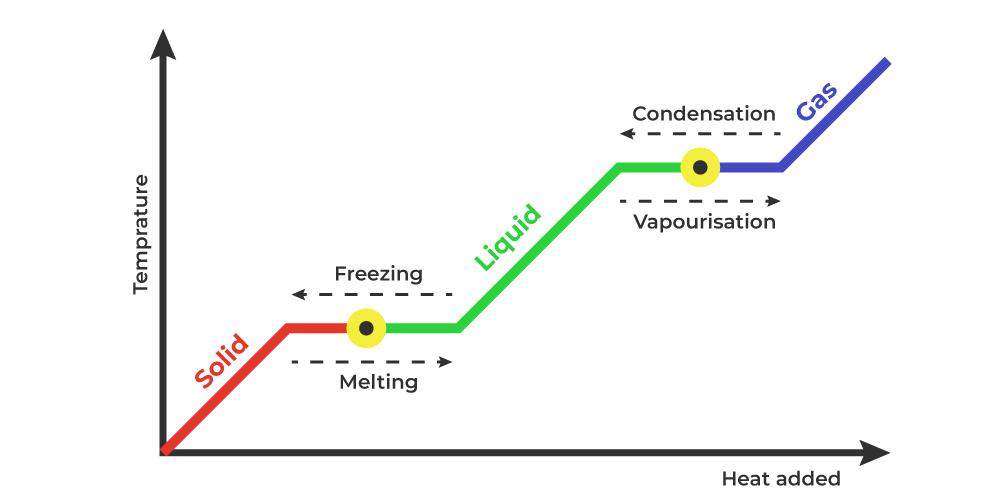
The study of the above graph tells us that,
The heat energy given to any solid substance increases its temperature until it reaches its melting point. At the melting point, the heat energy supplied does not change the temperature of the substance but the energy absorbed is required to change the phase of the substance. It changes from solid to liquid and the heat supplied is called the latent heat of fusion.
Similarly, the heat energy given to the liquid substance increases its temperature until it reaches its boiling point. At boiling point, the heat energy supplied does not change the temperature of the liquid but the energy absorbed is required to change the phase of the substance. It changes liquid to gas and the heat supplied is called the latent heat of vaporization.
Latent heat is called by various names depending upon the phase it changes.
- Solid-to-Liquid: Latent Heat of Fusion
- Liquid-to-Gas: Latent Heat of Vaporization
- Solid-to-Gas: Latent Heat of Sublimation
The formula for calculating the latent heat is given:
Q = ML
where,
L is Latent Heat
Q is Amount of Heat Released or Absorbed
M is Mass of Substance
The heat Q that must be supplied or withdrawn for an object of mass M to change phases is stated in this equation. Latent heat is denoted by the letter L.
Unit of Latent Heat
Latent heat is nothing but the heat required per kg to change the phase of any substance. Its unit is J⁄Kg
Dimension formula for latent heat is [M0L2T-2]
Type of Latent Heat
Depending on the state of change in the matter, latent heat is generally categorized into three categories, which include
- Latent Heat of Fusion
- Latent Heat of Vaporization
- Latent Heat of Sublimation
Latent Heat of Fusion
The heat consumed or emitted when matter melts, changing state from solid to fluid-structure at a constant temperature, is known as latent heat of fusion.
The heat energy required to transform a solid into a fluid at atmospheric pressure is the latent heat of fusion while the temperature remains constant during the operation. The enthalpy shift of any solid as it melts is known as the latent heat of fusion.
When the heat of fusion is expressed in terms of a unit of mass, it is referred to as the specific heat of fusion, whereas the molar heat of fusion refers to the enthalpy change per mole of material.
The inward energy of the fluid state is greater than that of the solid state. This means that energy must be delivered to the solid in order to dissolve it, and energy must be removed from a fluid when it solidifies because the particles in the fluid have a more fragile intermolecular force and so have larger potential energy (a sort of bond-separation energy for intermolecular powers).
Latent Heat of Vaporization
The heat consumed or expelled as matter disintegrates, changing phase from fluid to gas at a constant temperature, is known as latent heat of vaporization.
The heat of water vaporization is the most well-known. The heat of vaporization is defined as the amount of heat required to convert 1 g of a fluid into a fume without changing the fluid’s temperature. After a substance’s temperature has reached a breaking point, latent heat is necessary to change the state of the substance from fluid to gas at this point.
It’s worth noting that latent heat is associated with no change in temperature but a change in form. The disappearance of water has an obvious cooling effect, whereas the accumulation has a warming effect, due to the high heat of vaporization.
Latent Heat of Sublimation
When exposed to the open air, some chemicals, such as naphthalene, convert straight from solid to gas. The latent heat of sublimation is the amount of heat required for a substance to change from a solid to a gaseous state or the amount of heat required to remove heat from a gaseous material to turn into its solid state.
Check: Applications of Latent Heat in Daily Life
Specific Latent Heat
Specific latent heat is defined as the heat required to change the phase of one kg of any substance. It is similar to Latent Heat but the amount of substance is fixed to one kg.
The formula for specific latent heat is:
L = Q/m
where,
L is specific latent heat
Q is heat absorbed or released
m is mass of a substance
Sensible Heat
The heat transferred by a body or thermodynamic system that affects the temperature of the body or system, as well as some macroscopic variables of the body or system except pressure or volume, is called sensible heat.
Reasonable Heat and Meteorology
Meteorologists use reasonable heat to study the various parameters of the climate and predict the various natural events. When latent heat is released into the atmosphere it affects the climate. The reasonable heat absorbed and released into the atmosphere interacts with the hot or cold air and changes the climatic condition. conceivably delivering an extreme climate.
Example of Latent and Sensible Heat
We see various examples of latent heat in our daily life. Some of the most common examples of latent heat observed in our daily life are,
- The temperature of the steam continues to stay steady till all the water particle changes their state even though heat is supplied.
- The vertical movements of air or wind are caused by the latent heat released or absorbed by land or water.
- All the water freezes at the same temperature even though heat is continuously absorbed.
Also, Read
Solved Examples on Latent Heat
Example 1: Find the latent heat of a 20 kg substance if the amount of heat required for the phase change is 600 kcal.
Solution:
Given,
Q = 600 Kcal
M = 20 Kg
Formula for latent heat is given as:
L = Q ⁄ M
= 600 / 20
= 30 Kcal/Kg
Hence, the latent heat required for phase change is 30 Kcal / Kg.
Example 2: Find the mass of ice required to lower the temperature of 500 g of water at 20°C to water at 0°C. Given, Specific latent heat of ice is 336 J /g, the Specific heat capacity of water is 4.2 J/ g°C.
Solution:
Heat absorbed by Ice = Heat released by water
mIceL = mwaterc∆θ
Given,
mwater = 500 g = 0.5 kg
c = 4.2 J/ g°C
L = 336 J /g
∆θ = 20°C – 0°C = 20°C
mIce×336 = 500×4.2×20
mIce = (500×4.2×20) / 336
= 125 g
The required amount of ice is 125 gram
Latent Heat – FAQs
What is latent heat?
The amount of heat required to change the phase of any substance without changing its temperature is called latent heat.
What is an example of latent heat?
The temperature of the water remains at 100°C till the last drop evaporates even the though heat is supplied. The heat absorbed in this state is latent heat which changes the phase of water from liquid(water) to gas(steam)
What is specific latent heat?
The amount of heat required to change the phase of 1kg of any substance without changing its temperature is called the specific latent heat.
What are the types of latent heat transfer?
The two main types of latent heat transfer are
- Latent Heat of Fusion (Melting)
- Latent Heat of Vapourization (Boiling)
How latent heat can be measured?
Latent heat is measured by an instrument called Calorimeter.
What are Phase Changes?
When a substance undergoes change in its state it is called as phase change.
Why do we see water droplets on the outside of an ice-cold water-filled glass?
Water vapor is known to exist in the atmosphere. As a result, as air passes through the glass, the water vapor molecule collides with the cool surface and loses energy. Because when a material loses energy, it reverts to its original condition, water vapor becomes a liquid, and we observe water droplets on the glass’s outer surface.
Why is ice applied to a burned area of the skin?
The temperature of the wounded skin rises as a result of the burning. When ice is brushed against the skin, the surplus heat is absorbed by the substantial latent heat of fusion of water. As a result, the wounded skin’s temperature drops, and we experience less discomfort.
What are the types of latent heat?
There are two main types of latent heat: latent heat of fusion (involved in melting and freezing) and latent heat of vaporization (involved in evaporating and condensing).
How is latent heat calculated?
Latent heat is calculated by multiplying the mass of the substance undergoing the phase change by its specific latent heat (L), which is a property unique to each material and phase change.
What is an example of latent heat in daily life?
A common example of latent heat in everyday life is the cooling effect experienced when sweat evaporates from the skin. The body’s heat is used to evaporate the sweat, thereby cooling the body.
Why is latent heat important in refrigeration?
In refrigeration, latent heat is critical as it allows for the efficient transfer of heat out of the refrigerator or freezer compartment. The refrigerant absorbs latent heat when it evaporates, cooling the inside of the unit.
Can latent heat be negative?
Latent heat itself is not negative; however, the process of releasing latent heat (exothermic) can feel like cooling because heat is being removed from the surrounding environment.
What is the difference between latent heat and sensible heat?
Latent heat involves energy changes that do not affect temperature (associated with phase changes), while sensible heat refers to changes in temperature without a phase change.
Share your thoughts in the comments
Please Login to comment...