A solution is a homogeneous mixture of two or more substances. A solution is formed of two components: the solute and the solvent. The substance dissolved in the solution is called the solute, whereas the component in which the solute is dissolved is known as a solvent. In this article, we will learn about solutions and their types in detail.
What is a Solution?
A homogeneous mixture of two or more substances where the size of the particle is smaller than 1 nm is called the solution. Some common examples of solutions are sugar in water or salt in water solutions, etc. Unlike a mixture, the particle of solute in a mixture is evenly distributed. The solutions containing water as the solvent are called aqueous solutions. Solutions may contain solids, liquids or gases dissolved as solutes. The particles of a solution are not visible to the naked eye.
Components of Solution
Any solution has two basic components
Let’s learn about them in detail in this article.
What is a Solute?
The component of the solution that is dissolved in the other component is called the solute. Generally, the concentration of solute is low compared to the concentration of solvent.
What is a Solvent?
The component of the solution that dissolves a solute is the solvent. Solvents are generally in liquid or gaseous form. The concentration of solvent is high as compared to the concentration of solute.
Example in a solution such as lemonade sugar and lemon juice is considered solute, whereas water is considered a solvent. Water is considered the universal solvent.
Types of Solution
Solutions are divided into various categories based on the basis of various factors, like water as a solvent, amount of solute present, amount of solvent and the concentration of solute in two solutions. Let’s learn about each type in detail,
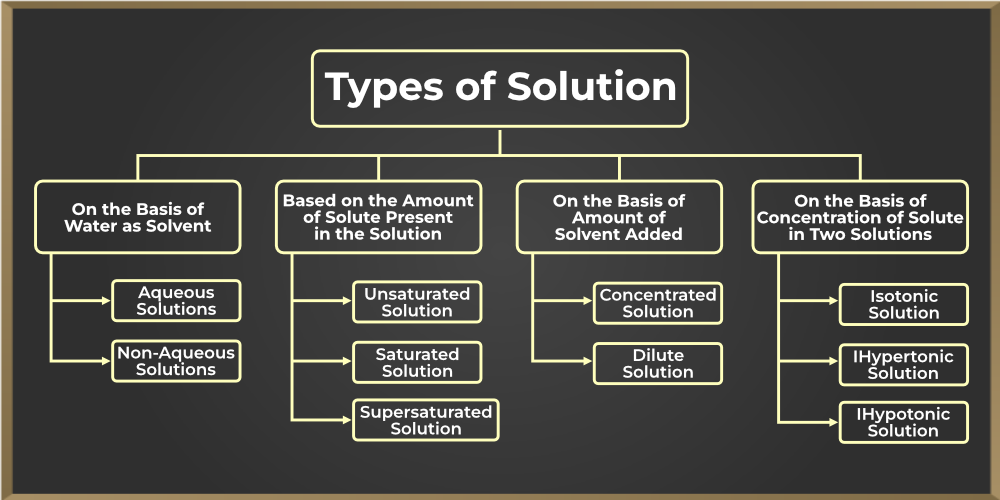
On Basis of Water as Solvent
The solutions can be divided into two categories depending on whether they contain water as the solvent or not which are,
- Aqueous Solutions
- Non-Aqueous Solutions
Now, let’s learn about them in detail,
Aqueous Solutions
Aqueous Solutions contain water as the solvent. Different solutes can be dissolved in water to form such solutions, such as salt water, sugar water or carbon dioxide in water.
Non-Aqueous Solutions
Non-Aqueous Solutions do not contain water as the solvent. The solvent could be other liquids such as ether, petrol, carbon tetrachloride, etc. Some examples of non-aqueous solutions are sulphur in carbon disulphide, naphthalene in benzene, etc.
On Basis of Amount of Solute
The solutions can be divided into three categories depending on the amount of solute present in the solution which are,
- Saturated Solutions
- Unsaturated Solutions
- Supersaturated solutions
Now, let’s learn about them in detail,
Saturated Solutions
A solvent can dissolve some particular types of solutes in it. The maximum amount of solute that can be dissolved in a solvent at a specified temperature can be termed a saturated solution. A solution cannot dissolve any more solute further upon reaching saturation. The undissolved substances remain at the bottom. The point at which the solute stops dissolving in the solvent is termed the saturation point.
Unsaturated Solutions
The amount of solute that is contained in lesser amounts than the maximum value, that is before the solution reaches the saturation level is called an unsaturated solution. No remaining substances leave at the bottom, that is, all the solute is dissolved in the solvent. An unsaturated solution is basically a chemical solution which has a solute concentration lesser than its corresponding equilibrium solubility.
Must Check
Supersaturated Solutions
The amount of solute contained in the solution exceeds the maximum amount of solute. The solution has already reached and crossed the saturation point. The solute is dissolved into the solution forcefully by raising the temperature or pressure of the solution. The solute particles on further dissolve, crystal out in the bottom of the container by the method called crystallization.
On Basis of Amount of Solvent
The solutions can be divided into two categories depending on the amount of solvent in the solution which are,
- Concentrated Solutions
- Dilute Solutions
Now, let’s learn about them in detail,
Concentrated Solutions
A concentrated solution contains large quantities of solute in the given solvent to form a solution. Some examples of concentrated solutions are mango juice, brine solution or dark colour tea.
Dilute Solutions
A dilute solution contains small quantities of solute in the given large quantity of solvent to form a solution. Some examples of dilute solutions are salt solutions or light colour tea.
On Basis of Concentration of Solute in Two Solutions
The solutions can be divided into three categories depending on the concentration of solute in the two solutions which include,
- Isotonic Solution
- Hypertonic Solution
- Hypotonic Solution
Let us consider a cell placed in a solution in a beaker. Based on the concentration of solute in two solvents(in the cell and in the beaker), we can have the following types of solutions:
Isotonic Solution
The solution contained in the beaker has a higher concentration of solute in it. As a result of this, the water emerges from the cell and into the solution contained in the beaker.
Hypertonic Solution
Hypertonic solutions contain the same concentration of solute in them. The water moves across the cell from the solution in the beaker in both directions.
Hypotonic Solution
There is a lower concentration of solute in the solution contained in the beaker. As a result, water goes into the cell which causes the cells to swell up and eventually burst.
Liquid solutions, such as sugar in water, are the most common kind, but there are also solutions that are gases or solids. Any state of matter (solid, liquid, or gas) can act both as a solute and as a solvent during the formation of a solution. Therefore, depending upon the physical states of solute and solvent, we can classify solutions into nine different types. The table given below tells us about all nine types of solutions.
| Types of Solution | Solute | Solvent | Examples
|
|---|
| Solid-Solid | Solid | Solid | Alloys like brass, bronze etc. |
| Solid-liquid | Solid | Liquid | The solution of sugar, salt etc in water. |
| Solid-Gas | Solid | Gas | Sublimation of substances like iodine, camphor etc into the air. |
| Liquid-Solid | Liquid | Solid | Hydrated salts, mercury in amalgamated zinc, etc. |
| Liquid-Liquid | Liquid | Liquid | Alcohol in water, benzene in toluene |
| Liquid-Gas | Liquid | Gas | Aerosol, water vapour in the air. |
| Gas-Solid | Gas | Solid | Hydrogen absorbed in palladium |
| Gas-Liquid | Gas | Liquid | Aerated drinks |
| Gas-Gas | Gas | Gas | A mixture of gases, etc |
For more details, check the Types of Solutions
Properties of Solution
Various properties of the solution are,
- Solution is a homogeneous mixture
- Solutions are generally very stable, which implies that the solute particles do not separate out on keeping.
- The particles dissolved are extremely small in size, generally less than 1 nm in diameter.
- Since the size of solute particles in the solutions is extremely small, they can easily pass through the filter paper. Therefore, components of a solution cannot be separated by filtration.
- The particles of a solution are negligibly small and cannot be seen even with a microscope.
- Since the size of solute particles in the solutions is extremely small, a true solution does not scatter light.
Examples of Solution
Some examples of Solutions are discussed below,
- Beverages like coffee or tea.
- Air is a solution containing gases primarily, Oxygen (21%) and Nitrogen (78%)
- The tincture of iodine contains alcohol as the solvent and iodine as the solute.
- Alloys are homogeneous mixtures of metals. For instance, brass is an alloy containing 30% zinc and 70% copper.
- Sugar syrup is a solution containing sugar dissolved as a solute in the solvent.
- Carbonated drinks contain solvent as water and carbon dioxide and other ingredients as solutes.
What is a Mixture?
When two or more substances are mixed together they form mixtures. In a mixture, the composition of the matter mixed is not always consistent. Some examples of the mixture include air, soil, sand, blood, etc. are different examples of mixtures. Mixtures are classified into two basic categories,
- Homogeneous Mixture: If the component of a mixture is distributed uniformly is it known as a homogeneous mixture.
- Heterogeneous Mixture: If the component of a mixture is distributed non-uniformly is it known as a heterogeneous mixture.
Must Check
Concentration of a Solution
Concentration of a Solution is defined as the amount of solute dissolved in the given solution. Depending upon the amount of solute dissolved in the solution, solutions are categorised as,
- Diluted Solutions
- Concentrated Solutions
- Saturated Solutions
Ways of Expressing Concentration
The formula for calculating the concentration of the solution is,
Concentration = (amount of solute)/(amount of solution)
Thus, we can say that the concentration of any solution is measured in grams/litres or kg/litre
Also, Check
Solved Examples on Solution
Example 1: 2ml of water is added to 4g of a powdered drug. The final volume is 3ml. Find the mass-by-volume percentage of the solution.
Solution:
Given,
Mass of solute = 4 g
Volume of solution = 3 ml
Mass by volume percentage = (Mass of solute) / (volume of solution) × 100 %.
= (4 / 3) × 100 %
= 133%.
Therefore, the mass by volume percentage is 133%.
Example 2: Many people use a solution of NaPO, to clean walls before putting up wallpaper. The recommended concentration is 1.7% (m/v). Find the mass of NaPO needed to make 2.0L of the solution.
Solution:
Given,
Concentration of solution = 1.7% (m/v) [mass by volume percentage]
Volume of Solution = 2 l = 2000 ml
Mass by volume percentage = (Mass of solute) / (volume of solution) × 100
1.7 = (Mass of solute) / (2000) × 100
Mass of solute = 34 g
Therefore the mass required is 34g.
Solution – FAQs
Write a short note on solutions with examples.
A homologous mixture of two or more substances where solute particles are smaller than 1 nm is called a solution. Sugar or salt dissolved in water is an example of the solution.
State the properties of the solution.
Some properties of the solution are:
- It is a homogeneous mixture.
- It is generally very stable in nature.
- The particle of the solution are very small and are not visible to the naked eye, etc.
What are the two classifications of solutions?
The two basic classifications of solution are,
- Aqueous Solution
- Non-aqueous Solution
What is the difference between a solute and a solution?
The substance which gets dissolved in a solution is called a solute whereas solvents are that substances which let solute to dissolve in them. For example, in a sugar solution with water, sugar is a solute whereas water is a solvent.
Give an example of a saturated solution.
If no further salt can be dissolved in a brine solution by physical means (without heating) then it is called a Saturated solution.
What exactly is a universal solvent?
Water is a universal solvent as it can dissolve a wide variety of solutes to form a solution.
Give examples of some solutions used in our daily life.
Some solutions used in our daily life are:
- Sugar in water solution.
- Salt in water solution (Brine solution).
- Alcohol dissolved in water.
Define the concentration of the solution.
The amount of solute present in a specified quantity of solution is referred to as the concentration of a solution.
Concentration of solution = [Tex]\frac{amountofsolute}{amountofsolution}[/Tex]
Define Aerosol.
A suspension which includes liquid droplets or fine solid particles as the solute dissolved in a gas as the solvent is called an aerosol. Examples : fog, mist or dust.
What is a true solution?
A True Solution is a homogeneous combination of two or more components immersed in a solvent with a particle size of less than 10-9 m or 1 nm. Example: The basic solution of sugar in water. By using filter paper that is often not noticeable to the naked eye, particles cannot be separated from real solutions.
Share your thoughts in the comments
Please Login to comment...