Matter is Made of Tiny Particles
Last Updated :
01 Apr, 2024
In our surroundings, we come across different shapes, sizes, heights, structures, and textures. According to scientists everything in this universe is made up of a material called Matter. We can see that matter occupies some space and mass, in another way we can say that matter has some ‘volume’ and ‘mass’.
However, in ancient times there was no clue to the scientists therefore at that time Indian Philosophers believed that everything in this universe either living or non-living can be divided into Panch Tattvas i.e. Air, Water, Fire, Sky, and Earth.
Chemistry is the study of matter’s composition and change. The substance is another term that is usually used equally with the matter, but it has a more limited definition in chemistry. Chemistry is the study of matter’s behavior. Chemistry is concerned with the composition, arrangement, and characteristics of matter, as well as the phenomena that occur when various types of matter change.
What is Matter
From a tiny needle to the highest statue of the world, everything is made up of matter. Matter is Made of Tiny Particles. In fact, everything in this Universe is a matter be it a drop of water, twinkling stars, or favorite food, but emotions like love, hate, hurt, etc. are feelings but do not matter.
In this article, we will understand the matter, its properties, and its constituents with a real example.
Every one of you must have seen Sunlight coming to your house through a small window, door, etc. Have you ever noticed the tiny particles that come along with sunlight? If, yes bravo because now you know that those tiny particles are called the matter and in case you have not noticed, please have a look because we human beings understand things easily with a practical example.
The matter is defined as everything that has mass and takes up space. Different types of matter include pens, paper, clips, sand, air, ice, and so on. Every substance is made up of microscopic particles.
These particles are so small that they are invisible to the human eye. Let’s look at the many properties of matter particles.
Nature of Particle
- A matter is made up of very tiny particles which may be atoms or molecules, for example- when we dissolve a spoon of salt or sugar in a glass of water.
- The matter is made up of particles– The particles of matter are very small beyond imagination these particles have space between them.
- Particles of matter are in continuous motion– This is because of the kinetic energy possessed by the particles which increases on increasing the temperature and so particles move much faster.
- Particles of matter attract each other by attractive forces– The attractive forces bind the particles of matter in a single body and lead to particles’ arrangement. The particles of matter have a force acting between them.
Important Point: Both sugar and salt are made up of very small particle which break off from each sugar granule and get disappeared in water on dissolving, that’s why solution tastes sweet, but particle cannot be seen, this shows that water have enough space to adjust (dissolve) tiny sugar or salt particle.
Characteristics of Particles of Matter
- Particles of matter have space between them: This property simply explains that the particles of matter are packed but still there is a slight amount of space between them and due to which it can be categorized into solid, liquid, and gas.
- Particles of matter are continuously moving: This property means that the particles are not at rest, they are continuously moving and colliding and producing Kinetic energy.
- Particles of matter attract each other: This property of matter explains that particles of matter attract each other.
Also Read
Classification Of Matter
From early times, we human beings are researching to create a basic understanding of the surroundings. Centuries ago, Indian philosophers classified matter into five elements i.e the Panch Tatva– air, earth, fire, sky, and water.
They stated that everything, living or nonliving, was made up of these five basic elements. Ancient Greek philosophers had arrived at a similar classification of matter.
Modern-day scientists have evolved two types of classification of matter based on their physical properties and chemical nature.
Must Check
(1) Classification Based on Physical Nature
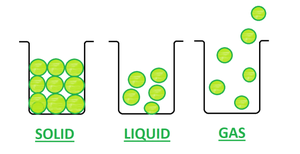
- Solid– In Solid, the particles are very closely packed. And, thus the force of attraction between particles is very strong. Due to its closely packed nature, it has a definite shape and volume. The solids have a high density and can not be diffused. A rubber band is a solid because it can change its shape under force and regain its shape when force is removed if excessive force is applied it breaks. This is an exceptional case of the solid.
- Liquid– In Liquids, the particles are less closely packed as compared to solid. And, thus the force of attraction between particles is also less strong. They do not have a definite shape but have a definite volume. Also, Density is lower than solids and can diffuse.
- Gas– In Gas, the particles are far apart from each other. The Force of attraction between particles is negligible. Gas neither has a definite shape nor definite volume. Density is least and can easily diffuse.
- Plasma- Plasma is considered the fourth state of matter. Plasma is a mixture of free electrons and ions. It occurs naturally in the stars. Inside the stars, the temperature is so high that the atoms break down. And, this mixture of free electrons and ions in a state is called plasma. The sun and the other Stars glow because of the pressure of Plasma in them. Plasma can also be made on the earth by passing electricity through gases at less temperature plasma make the fluorescent tube glow.
- B.E. Condensate(Bose-Einstein)- In 1921 Indian scientist Satyendra Nath Bose did some calculations for the fifth state of matter. Based on their calculation, Albert Einstein predicted the existence of a new state of matter. The fifth state of matter was achieved finally by three scientists Cornell, Keller, and Wieman of the USA cooling gas in extremely low density to super low temperature.
Now, let’s study the properties of the three major states of the matter in detail.
Properties of Solid
- Definite shape, size, volume, and distinct boundaries.
- Negligible compressibility.
- Solids tend to maintain their shape when subjected to outside force.
- They are rigid, difficult to change their shape.
- Mass per unit volume of a substance is called density.
- Intermolecular force is high in solids.
- Kinetic energy is very low in solids.
- Solids do not possess the property of diffusion.
Properties of Liquid
- Do not have a definite shape or distinct boundaries but have a fixed volume.
- They can be compressed.
- In Science the common name of gases and liquids is fluid.
- Liquids are not Rigid but have the property of flowing that’s why liquids are called fluids.
- Intermolecular force is less than the solids.
- In liquid kinetic energy is more than solid.
- Liquid has moderate density.
- Liquids possess the property of diffusion.
- They can take any shape.
- The gases Oxygen and Carbon dioxide from the atmosphere diffuse and dissolve in water. Due to these gases, aquatic plants and animals can survive.
- Diffusion is much more in liquid than in solid due to the free movement of particles of liquid.
Also Read
Properties of Gas
- Gas is neither a definite shape nor a definite volume.
- They can be compressed much.
- Gases have maximum fluidity and less rigidity.
- The intermolecular force of attraction is the least.
- The kinetic energy of its particles is maximum.
- Gases have no density.
- They can take any shape.
- Very fast diffusion.
- The particles in a gas are free to move and hence gases can flow in any direction. They occupy all the volume available to them.
- LPG = Liquefied Petroleum Gas and CNG = Compressed Natural Gas that is fuel in vehicles.
Also Read
(2) Classification Based on Chemical Nature
- Mixture: Whenever two or more substances mix without engaging in a chemical process, the resulting product is called a Mixture. For e.g Seawater is a mixture of various salt and water.
- Pure Substances- Substances that are made up of some kind of particles and have a fixed composition. E.g silver, copper, etc.
Sample Problems
1. How Small are particles of Matter?
These particles are very small even beyond our imagination, and they cannot be seen from naked eyes. In simple words, the matter is something that has mass and occupies space. The matter is a combination of particles.
2. What do you mean by matter?
The matter is something that has mass and which occupies space. Based, on the physical and chemical nature of matter. The matter can be classified as solid, liquid, and gas based on its physical nature. On the other hand, based on chemical nature, they are classified as mixtures and Pure substances.
3. Mention some characteristics of matter.
The characteristics are as follows:-
- The Particles of matter have space between them.
- The Particles of matter are continuously moving.
- The Particles of matter attract each other.
4. When 50 g of salt is dissolved in 100 mL of water, there is no increase in volume. What characteristic of matter is illustrated by this observation?
This observation indicates that particles of water have spaces between them into which sugar particles fit.
5. Gases fill the vessel in which they are kept. Give reasons.
In the gaseous state, particles move freely and have greater space between them. So they occupy the entire space available. Hence, gases fill the vessel in which they are kept.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...