Atomic mass is the total mass of all subatomic particles of an atom, including protons, neutrons, and electrons. However, the mass of electrons is incredibly small, so it’s typically neglected when determining an atom’s overall mass.
The unit commonly used to express atomic mass is the Atomic Mass Unit (AMU) and dalton, a non-SI unit. One dalton is equivalent to one-twelfth of the mass of a carbon-12 atom at rest in its ground state. This definition provides a standard reference point for measuring atomic masses.
The atomic mass of an individual atom is closely related to its mass number, which represents the total number of protons and neutrons in the nucleus. Since protons and neutrons contribute most to an atom’s mass, the atomic mass is nearly identical to the mass number. This relationship helps simplify calculations and understanding of atomic masses.
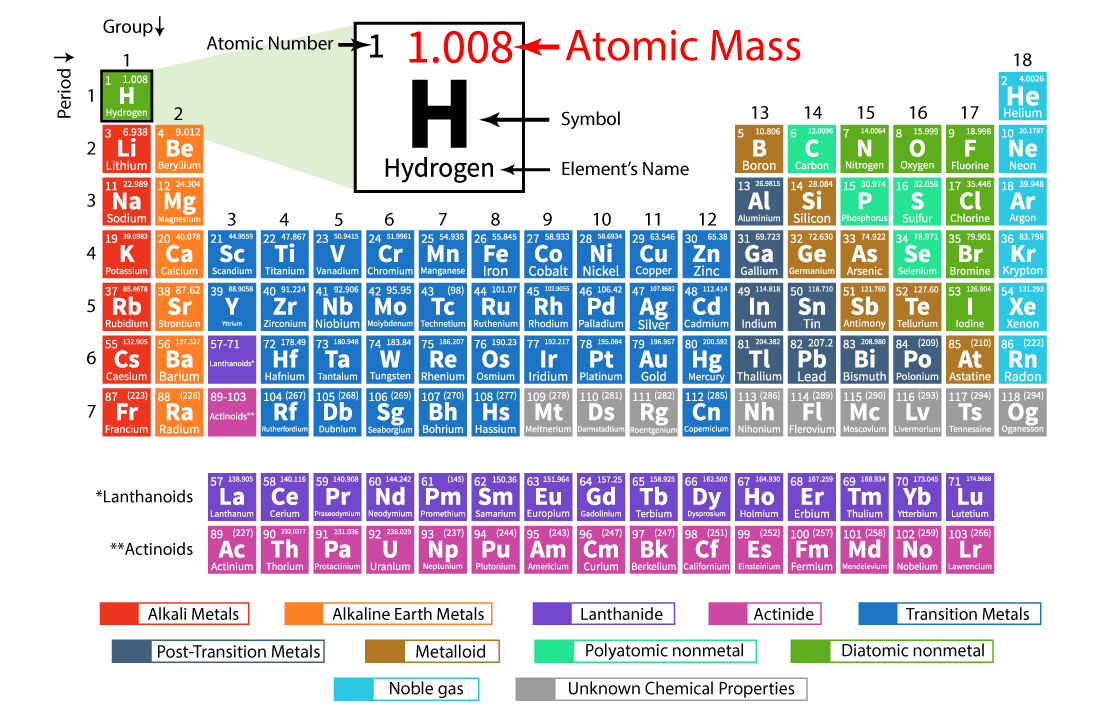
Atomic Mass Definition
Atomic Mass of an element is a measure of the average mass of its atoms. It is expressed in Atomic Mass Units (AMU).
Atomic mass of an element is defined as the total mass of one atom of that element.
The average mass of an element’s atoms expressed in atomic mass units is known as its atomic mass (amu, also known as daltons, D). The atomic number can also be called atomic weight.
Atomic Mass Unit
An atomic mass unit (amu) is a unit of mass used to express atomic and molecular mass. It is defined as one twelfth (1/12) of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state. It is equivalent to approximately 1.66053906660 × 10-27 kilograms.
Atomic Mass of First 30 Elements
Here is the table showing the first 30 elements of the periodic table with their atomic masses and atomic number:
|
List of Atomic Mass of First 30 Elements
|
|
Atomic Number
|
Elements
|
Symbol
|
Atomic Mass (in amu)
|
|
1
|
Hydrogen
|
H
|
1.008
|
|
2
|
Helium
|
He
|
4.0026
|
|
3
|
Lithium
|
Li
|
6.94
|
|
4
|
Beryllium
|
Be
|
9.0122
|
|
5
|
Boron
|
B
|
10.81
|
|
6
|
Carbon
|
C
|
12.011
|
|
7
|
Nitrogen
|
N
|
14.007
|
|
8
|
Oxygen
|
O
|
15.999
|
|
9
|
Fluorine
|
F
|
18.998
|
|
10
|
Neon
|
Ne
|
20.18
|
|
11
|
Sodium
|
Na
|
22.99
|
|
12
|
Magnesium
|
Mg
|
24.305
|
|
13
|
Aluminium
|
Al
|
26.982
|
|
14
|
Silicon
|
Si
|
28.085
|
|
15
|
Phosphorus
|
P
|
30.974
|
|
16
|
Sulfur
|
S
|
32.06
|
|
17
|
Chlorine
|
Cl
|
35.45
|
|
18
|
Argon
|
Ar
|
39.948
|
|
19
|
Potassium
|
K
|
39.098
|
|
20
|
Calcium
|
Ca
|
40.078
|
|
21
|
Scandium
|
Sc
|
44.956
|
|
22
|
Titanium
|
Ti
|
47.867
|
|
23
|
Vanadium
|
V
|
50.942
|
|
24
|
Chromium
|
Cr
|
51.996
|
|
25
|
Manganese
|
Mn
|
54.938
|
|
26
|
Iron
|
Fe
|
55.845
|
|
27
|
Cobalt
|
Co
|
58.933
|
|
28
|
Nickel
|
Ni
|
58.693
|
|
29
|
Copper
|
Cu
|
63.546
|
|
30
|
Zinc
|
Zn
|
65.38
|
Mass Number
The total number of protons and neutrons in an atom’s nucleus is known as the mass number. Consequently, an atom of carbon will have an exact mass of 12 if its nucleus contains 6 protons and 6 neutrons.
The difference between the mass number and the atomic mass of an element is that a mass number is a whole number obtained by the addition of a number of protons and neutrons. While the atomic mass or atomic weight is the average number of protons and neutrons in an element for all of its isotopes.
The formula to determine the mass number of an atom of a specific element is given by :
Mass Number = Number of Protons + Number of Neutrons
How to Calculate Atomic Mass?
Here are three ways to determine the Atomic Mass, depending on the given conditions:
- By looking up to the atomic mass on the periodic table- In the periodic table digit of an atomic mass is usually marked under the representation of an element. Like Hydrogen 1u, Helium 3u.
- Adding the Mass of Protons and Neutrons – The total number of protons and neutrons in an atomic nucleus gives the mass number.
- Weighted Average for All Atoms of an Element – The weighted average of each element’s isotopes, grounded in nature by their abundance, is the atomic weight of that element.
These recommendations can be used to calculate the atomic mass of the component. A list of isotopes with natural abundance and mass is given a percentage or decimal value. Each isotope’s abundance is multiplied by its mass. Divide the solution by 100 and add the findings if isotope abundance is present.
Differences Between Atomic Mass and Atomic Number
The 5 key differences between atomic mass and atomic number are as follows:
- Definition:
- Atomic Mass: Atomic mass refers to the total mass of all particles (protons, neutrons, and electrons) present in an atom.
- Atomic Number: Atomic number represents the number of protons in the nucleus of an atom.
- Units:
- Atomic Mass: Atomic mass is measured in atomic mass units (amu) or daltons.
- Atomic Number: Atomic number is a dimensionless quantity and is simply represented by a whole number.
- Composition:
- Atomic Mass: The atomic mass includes the combined mass of protons, neutrons, and electrons within an atom.
- Atomic Number: The atomic number specifically counts the number of protons in the nucleus of an atom.
- Relation to Isotopes:
- Atomic Mass: Atomic mass takes into account the contributions of different isotopes of an element, considering their abundance and the mass of each isotope.
- Atomic Number: Atomic number remains constant for a specific element and does not change with different isotopes. It uniquely identifies the element.
- Role in Periodic Table:
- Atomic Mass: Atomic mass contributes to the average atomic mass of elements as seen in the periodic table. It helps in understanding the distribution of isotopes and their relative abundances.
- Atomic Number: Atomic number is the basis for the arrangement of elements in the periodic table. Elements are ordered by increasing atomic number, which reflects the number of protons and defines the element’s identity.
Fractional Atomic Mass
Fractional atomic mass refers to the average mass of an element’s isotopes relative to the mass of a carbon-12 atom, which is assigned a mass of exactly 12 atomic mass units (amu). It is expressed as a decimal fraction.
The fractional atomic mass takes into account the relative abundances of the isotopes of an element found in nature. Since many elements exist as a mixture of isotopes, each with its own mass and abundance, the fractional atomic mass provides a weighted average of these isotopic masses.
For example, carbon has two stable isotopes: carbon-12 and carbon-13, with natural abundances of approximately 98.9% and 1.1%, respectively. To calculate the fractional atomic mass of carbon, one would consider the mass of each isotope weighted by its abundance:
Fractional Atomic Mass of Carbon = (0.989 × 12) + (0.011 × 13)
This calculation yields the average atomic mass of carbon, taking into account the contributions of both isotopes based on their natural abundance.
Fractional atomic masses are essential for accurately representing the atomic masses of elements in the periodic table, which provides valuable information for chemical calculations and understanding the properties of elements.
Molecular Mass
The atomic masses of the constituent atoms that make up a compound are added to determine its molecular mass.
The mass a molecule carries is known as its molecular mass, additionally known as molecular weight. It can be calculated by summing the masses of all the atoms in the molecule and multiplying the result by the number of the element’s atoms.
One oxygen atom and two hydrogen atoms, for instance, make up water. A water molecule’s mass is determined by multiplying its average hydrogen atomic mass by two and adding its average oxygen atomic mass. The atoms that make up the molecule are what define an element’s molecular mass.
Atomic Number
An element’s atomic number is determined by how many protons are found in one of its atoms. Each atom in an element has precisely the same number of protons. For instance, the atomic number of carbon is “6” since all carbon atoms have six protons.
Regardless of the presence of neutrons, elements are classified according to the number of protons in their nuclei. Uncharged subatomic particles known as neutrons are stable when contained in an atomic nucleus. Except for hydrogen, all other elements’ atoms have neutrons in the nucleus. Hence,
Atomic Number = Number of Protons in an atom
Quiz on Atomic Mass
Practice problems on atomic mass in our carefully curated quiz. Find important questions, their answers and explanations too.
FAQs on Atomic Mass
What is Atomic Mass?
The mass of a single atom in a chemical element is known as its atomic mass. It involves the masses of protons, neutrons, and electrons, the three atomic subatomic particles.
What is 1 amu?
1 a.m.u is defined as the average of the rest mass of proton and neutron, and is equal to 1.67377 × 10-27 kg.
What is Atomic Mass Formula?
The atomic mass of an element is calculated using the formula:
Atomic Mass = ∑ (Isotope Mass × Isotope Abundance)
Where:
- Isotope Mass is the mass of an individual isotope of the element.
- Isotope Abundance is the natural abundance of that isotope expressed as a decimal (for example, an abundance of 50% would be expressed as 0.50).
Why was Carbon-12 selected as Reference Element for Calculating Atomic Mass?
Because it is the only atom with the same whole-number mass as the amu scale, carbon-12 is utilised in amu.
What is an Atom Made Of?
The three main types of particles that make up an atom are protons, electrons, and neutrons.
What is Atomic Mass Number?
The total number of protons and neutrons (together known as nucleons) in an atomic nucleus is the mass number.
What is Atomic Mass of Carbon?
The atomic mass of carbon is approximately 12.01 atomic mass units (amu).
What is Atomic Mass of Sulphur?
The atomic mass of sulphur is approximately 32.06 atomic mass units (amu).
What is Atomic Mass of Oxygen?
The atomic mass of oxygen is approximately 15.999 atomic mass units (amu).
What is Atomic Mass of Helium?
The atomic mass of helium is approximately 4.002602 atomic mass units (amu).
What is Atomic Mass of Hydrogen?
The atomic mass of hydrogen is approximately 1.008 atomic mass units (amu).
What is Atomic Mass of Sodium?
Atomic mass of Sodium is 23u (22.990u).
Share your thoughts in the comments
Please Login to comment...