Factors Affecting Enzyme Activity
Last Updated :
08 Apr, 2024
The factors affecting enzyme activity include temperature, pH, substrate concentration, enzyme concentration, and the presence of inhibitors or activators. Enzymes are biological catalysts that accelerate chemical reactions in living organisms. Enzymes are made up of proteins that are highly specific and crucial for physiological processes.
Understanding these factors is essential for optimizing enzyme function and maintaining biological processes in balance. In this article, you will factors affecting enzyme activity notes.
Enzymes Meaning and Definition
Enzyme Definition: Enzymes are protein molecules that functions as biological catalysts and helps accelerate chemical reactions essential for life.
Most enzymes are made up of proteins except ribozymes. All metabolic pathway processes are catalysed by intracellular enzymes. They play a crucial role in various bodily functions including digestion and metabolism. Enzymes function by lowering the activation energy required for a reaction to occur, allowing it to happen more quickly and efficiently. Enzymes degrade substrates into simpler molecules known as products. Each enzyme is highly specific, recognizing and binding to a particular substrate molecule to catalyze a specific reaction.
What are the Factors Affecting Enzyme Activity?
The reaction conditions have a significant impact on enzyme activity. Enzymes functions effectively under the optimal conditions. Enzyme activity can be influenced by various factors such as temperature, pH, enzyme concentration, substrate concentration, inhibitor, or accumulation of end products. These factors can alter the shape and structure of the enzyme, affecting its ability to bind to the substrate and catalyze the reaction efficiently. These factors are discussed below:

Factors Affecting Enzyme Activity
Effect of Temperature on Enzyme Activity
- The kinetic energy of molecules increases with increasing temperature. This means that there are more random collisions between molecules per unit of time in a fluid.
- Because enzymes catalyze reactions by randomly colliding with substrate molecules, increasing the temperature speeds up the reaction, resulting in more product formation.
- As the temperature rises, more bonds, particularly the weaker hydrogen and ionic bonds, will break due to strain. When bonds within the enzyme are broken, the active site changes shape.
- However, increasing the temperature increases the vibrational energy of molecules, specifically enzyme molecules, putting strain on the bonds that hold them together.
- As temperature rises, the shapes of more enzyme molecules’ active sites become less complementary to the shape of their substrate, and more enzymes are denatured. This reduces the rate of reaction.
- As the active site is no longer complementary to the shape of the substrate, it is less likely to catalyze the reaction. The enzyme will eventually become denatured and cease to function.
- As temperature rises, the rate of reaction rises initially due to increased kinetic energy. However, the effect of bond breaking will increase over time, and the rate of reaction will begin to slow.
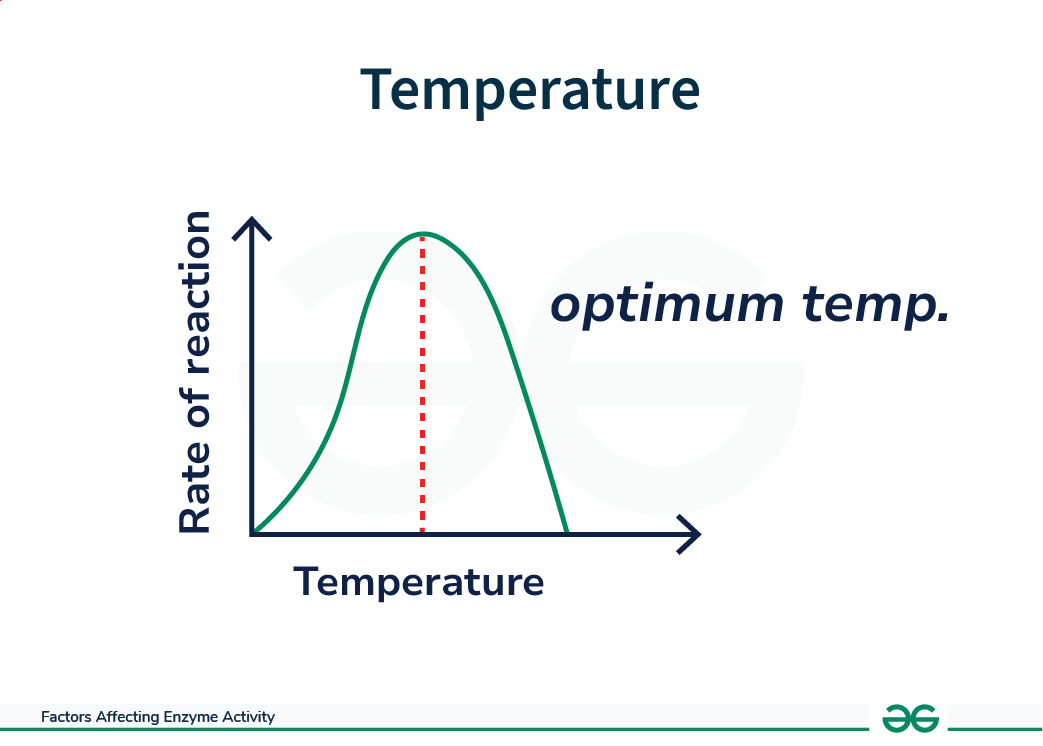
Effect of Temperature on Enzyme Activity
Effect of Enzyme Concentration on Enzyme Activity
- Enzyme concentration directly affects enzyme activity by influencing the number of enzyme molecules available to catalyze reactions.
- Increasing enzyme concentration leads to more active sites available for substrate binding, thereby increasing the rate of reaction.
- This occurs because higher enzyme concentrations result in more collisions between enzymes and substrates, leading to more successful enzyme-substrate complexes being formed.
- However, once all substrate molecules are bound to enzymes, further increases in enzyme concentration will not significantly affect the reaction rate, as the active sites become saturated.
- Conversely, decreasing enzyme concentration leads to fewer active sites available for substrate binding, resulting in a slower rate of reaction.
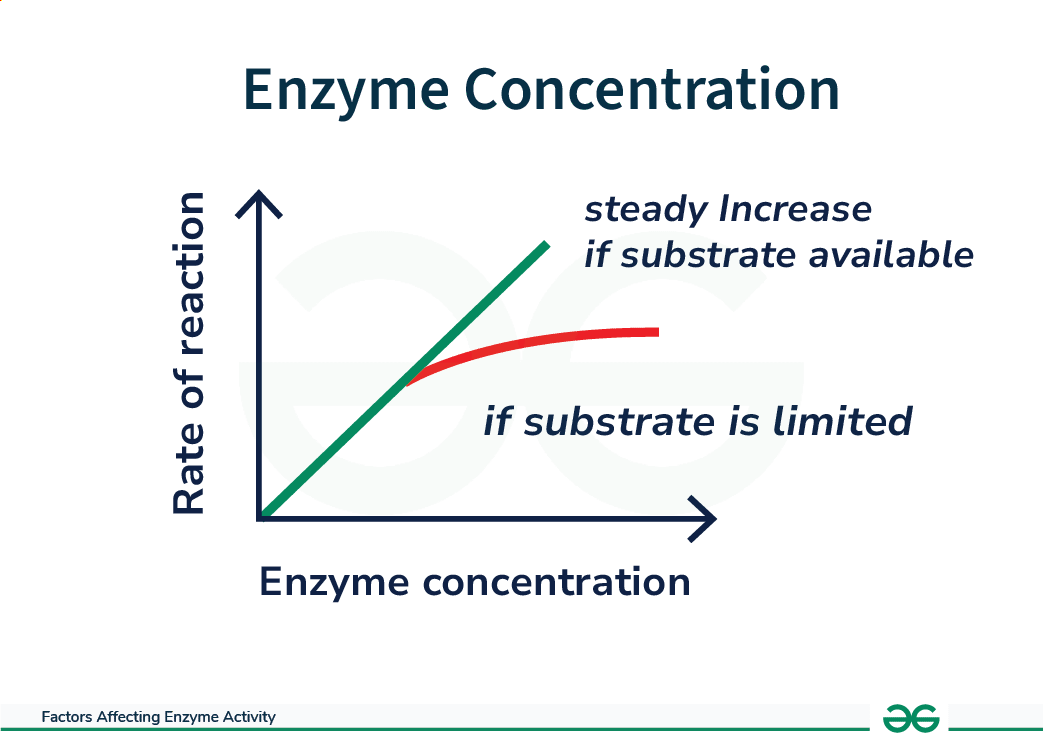
Effect of Enzymes Concentration on Enzyme Activity
Effect of Substrate Concentration on Enzyme Activity
- Substrate concentration directly influences enzyme activity by affecting the rate of enzyme-substrate complex formation.
- Initially, as substrate concentration increases, the rate of reaction also increases because more substrate molecules are available to bind with enzyme active sites.
- This occurs until all enzyme active sites are saturated with substrate, leading to maximum reaction velocity (Vmax).
- At this point, further increases in substrate concentration do not increase the reaction rate because all enzyme active sites are already engaged.
- Conversely, when substrate concentration decreases, fewer substrate molecules are available to bind with enzyme active sites, resulting in a slower rate of reaction.

Effect of Substrates Concentration on Enzyme Activity
Effect of pH Value on Enzyme Activity
- Proteins, like enzymes, contain electrical charges due to the sequence of their amino acid residues because they are made up of a chain of amino acids.
- The majority of the amino acids in the chain serve as the foundation for intramolecular interactions that give the enzyme its three-dimensional structure. Few others serve as functional residues at the active site of the enzyme.
- Overall, the amino acids determine substrate specificity and limit enzyme activity to a narrow pH range. Most enzymes work best in slightly acidic or basic environments.
- However, because some enzymes are native to extremely acidic or basic environments, they are most active in these pH ranges.

Effect of pH Value on Enzyme Activity
Effect of Inhibitor or Effector on Enzyme Activity
- Many enzymes depend on non-substrate and non-enzyme molecules to regulate or initiate catalysis. Certain enzymes, for example, depend on metal ions or cofactors to establish catalytic activity.
- Many enzymes, such as allosteric enzymes, rely on effectors to activate their catalytic activities and promote or inhibit their subsequent binding to substrates.
- Similarly, inhibitors may bind to the enzyme or its substrate in order to inhibit ongoing enzymatic activity and prevent subsequent catalytic events.
- When inhibitors form strong bonds to the enzyme’s functional group, the effect on enzyme activity is irreversible, rendering the enzyme permanently inactive.
- Reversible inhibitors, as opposed to irreversible inhibitors, only render enzymes inactive when bound to the enzyme.
- Competitive inhibitors compete with substrates for binding to the enzyme functional group residues at the catalytic sites. Other types of inhibitors bind to the non-substrate binding allosteric site rather than the catalytic site.
- It is non-competitive if an inhibitor binds to the enzyme concurrently with the enzyme-substrate binding. An inhibitor is uncompetitive if it only binds to a substrate-occupied enzyme.
Effect of Accumulation of End-Products on Enzyme Activity
- The accumulation of end-products can inhibit enzyme activity through feedback inhibition which is a regulatory mechanism that helps maintain homeostasis.
- When end-products accumulate to high levels, they can bind to the enzyme’s allosteric site, altering its conformation and reducing its catalytic activity.
- This inhibition prevents excessive production of end-products and conserves resources within the cell.
- As end-product levels decrease, inhibition is removed. This allows enzyme activity to resume.
Conclusion – Factors Affecting Enzyme Activity
In conclusion, enzymes are biological catalysts that regulate chemical reactions in living organisms. There are various factors affecting enzyme activity, including temperature, pH, substrate and enzyme concentrations, and the presence of inhibitors or activators. Understanding these factors is crucial for optimizing enzyme function and maintaining biological processes in balance. By understanding the complex interaction between enzymes and their environment, scientists can develop strategies to enhance enzyme performance and address challenges in diverse fields, from medicine to biotechnology.
Also Read:
FAQs on Factors Affecting Enzyme Activity
Explain the Enzyme Concentration Factor that Influences Enzyme Activity.
Enzyme concentration directly impacts enzyme activity by altering the number of available enzyme molecules for catalysis, thereby affecting the rate of reaction.
Why does pH Affect Enzyme Activity?
pH affects enzyme activity by influencing the charges on amino acid residues within the enzyme’s active site, which can disrupt substrate binding and alter enzyme structure.
What is the Structure of an Enzyme?
Enzymes typically have a globular protein structure, characterized by a specific three-dimensional shape that includes an active site where substrates bind.
What are the Factors that Influence Enzyme Activity?
Factors influencing enzyme activity include temperature, pH, substrate concentration, enzyme concentration, cofactors, inhibitors, and activators.
What are the functions of Enzymes?
Enzymes facilitate biochemical reactions by lowering the activation energy required for the reaction to proceed, thereby increasing the reaction rate.
What are the 7 Factors that Affect the Enzyme Activity?
The seven factors that affect enzyme activity are temperature, pH, substrate concentration, enzyme concentration, presence of inhibitors or activators, cofactors, and substrate specificity.
Share your thoughts in the comments
Please Login to comment...