Chromatography is the separation of mixtures by distribution between two or more immiscible phases. In chemical laboratories, where it is used for analysis, isolation, and purification, chromatography is the most used separation technique. It is also frequently used in the chemical process industry as a part of small and large-scale production.
There are many types of Chromatography, e.g. Adsorption Chromatography, Thin Layer Chromatography, Column Chromatography, Partition Chromatography, etc. Paper Chromatography is an example of Partition Chromatography. Let’s learn more about Chromatography, its meaning, principle, types, and applications along with FAQs on them, in this article!
Chromatography Definition
Chromatography is a technique used to affect the separation of two or more dissolved solids contained within a solution in very small quantities. In Greek, the word ‘chroma’ means colour and ‘graphein’ is used to indicate writing. Initially, the technique was used for the separation of colors.
The substance that has to be segregated during the process of chromatography is known as an analyte. It basically refers to the component needed from the mixture. It is a physical process in which the solutes, that is the components of a sample mixture are segregated as a result of their differential distribution between the stationary and mobile phases.

Terms used in Chromatography
Let’s learn some of the terms that are frequently used in chromatography
- Analyte: Analyte is the substance that is to be separated from the mixture during chromatography.
- Mobile Phase: Mobile Phase in Chromatography is the component that moves with the sample. It is either a gas or a liquid and is passed through the column where the components of the mixture are absorbed.
- Stationary Phase: Stationary Phase in Chromatography is the one that doesn’t move with the sample. It is generally a porous solid that absorbs components from the mobile phase.
- Eluent: Eluent refers to the fluid that enters and passes through the chromatographic column.
- Elute: Elute is the fluid containing the sample that exits the chromatographic column
- Elution: Elution is the process of removal or extraction of a solid by washing out it with a suitable solvent in a chromatographic column.
Also Read: Separation of Mixtures
Principles of Chromatography
The separation technique of chromatography is dependent on the principle of partitioning the constituent components between two phases – a Mobile Phase and a Stationary Phase. The mobile phase is used to indicate the mixture of the substances to be separated and dissolved in a liquid or a gas. The stationary phase is used to describe a porous solid matrix through which the sample present in the mobile phase percolates.
The method of chromatography is based on the fact that the dissolved substances in the same solvent may have differing solubility. The solute with a greater solubility rises faster and segregates from the mixture.
The different solubility of the analyte is due to the differential affinity or the different strength of adhesion towards the mobile and stationary phases. The affinity of an analyte can be explained by two terms, the first is adsorption and the second is solubility. Adsorption is the ability to get attached to the surface of the stationary phase while solubility is the ability to dissolve in the mobile phase.
Analyte having higher adsorption will slowly pass through the column while Analyte having high solubility will move faster through the column. The difference in the solubility and adsorption is due to the difference in polarity towards different phases which determines the extent of adsorption and solubility. For Example, a polar analyte will adsorb better to a polar stationary phase thus moving slowly through the column, and a non-polar analyte will better dissolve in a non-polar mobile phase thus moving faster through the column.
Read More:
Types of Chromatography
Chromatography can be classified into different types based on the following categories based on the physical state of mobile and stationary phases. The different types of Chromatography are mentioned below:
- Adsorption Chromatography
- Thin Layer Chromatography
- High-Performance Liquid Chromatography
- Column Chromatography
- Partition Chromatography
- Gel Filtration Chromatography
- Liquid Chromatography
- Gas Chromatography
- Gas Liquid Chromatography
Adsorption Chromatography
Adsorption Chromatography is based on the differential rate of adsorption of the solute to the stationary phase. In Adsorption Chromatography the mobile phase is liquid or gas in which solute is dissolved. Depending on the component’s absorptivity, different solutes are adsorbed on the adsorbent to varying degrees in the adsorption chromatography process. The components with higher absorptivity are carried to a shorter distance than those with lower absorptivity in this case as well by moving a mobile phase over a stationary phase. Adsorption Chromatography is used in the identification of carbohydrates, and fats, separation and isolation of amino acids, antibiotics, etc.
Thin Layer Chromatography
Thin Layer Chromatography also called TLC Chromatography is used to separate non-volatile liquids using a thin stationary phase. As shown in the image above, the thin-layer chromatography (TLC) procedure uses a glass plate coated with a very thin layer of an adsorbent, such as silica gel or alumina, to separate the mixture of chemicals into its constituent parts.

Chrome Plate is the name of the plate used in this technique. A small area of the mixture’s solution is put 2 cm above one end of the plate to begin the separation process. The plate is then placed in a sealed container filled with an eluting fluid, which rises up the plate while lifting various mixture ingredients to various heights. The heights to which components rise are called spots which appear separately in a vertical arrangement. Each spot is characterized by a factor called as Retention Factor which is given by the formula mentioned below:
Retention Factor(Rf) = Distance Travelled By Sample/Distance Travelled by Solvent
Learn More, Retention Factor
High-Performance Liquid Chromatography
High-Performance Liquid Chromatography also called HPLC Chromatography is a method in analytical chemistry to separate the components of a mixture and to identify and measure their quantity. This method was introduced by M.S. Tswett who was a botanist. A sample has two types of components named analyte and matrix. It is the analyte that we want to identify and quantify and the rest of the other components of the sample are called the Matrix.
In chromatography, the sample is introduced in the mobile phase which is a liquid that passes through a solid which is usually a solid or a liquid on the solid. In HPLC, the stationary phase is in a column packed with small porous particles and the liquid passes through it with the help of a pump. The sample then passes through the column. The particles of the sample move at different rates due to the varying nature of interaction with the stationary phase. After the sample crossed the column, the substances are identified and quantified using the computer and the software attached to it.

The different types of HPLC include Normal Phase HPLC, Reverse Phase HPLC, Ion Exchange HPLC, and Size Exclusion HPLC.
HPLC is used in the analysis of drugs, synthetic polymers, environmental particles, water purification, and isolation of various valuable products.
Column Chromatography
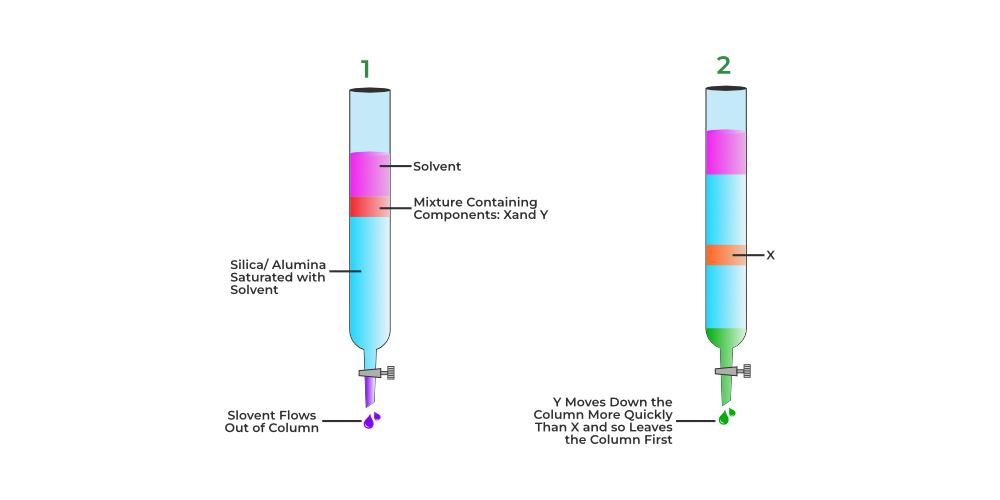
Column Chromatography is used to separate a single compound dissolved in a chemical mixture. Column Chromatography is based on the principle of differential rate of adsorption. In this method, a Column is prepared by filling the tube with a solid porous absorbent which is the stationary phase. A fluid containing the solute is passed through this column. The fluid entering the column is called eluent and in between the column solute gets adsorbed after that the fluid exits the column which is called the elute.

As shown in the figure above, column chromatography is a technique used to separate the components of a mixture using a column of appropriate adsorbent packed in a glass tube. A suitable eluant is produced to flow gently down the column in drop by drop manner after the mixture is placed on top of it.
The components are separated based on how much of each component has been adsorbed to the wall of the adsorbent column. The element with the greatest absorptivity is kept at the top while the other elements flow downward to various heights in accordance.
Column Chromatography is a low-cost technique used to isolate active ingredients from a compound, remove impurities, and in drug estimation.
Paper Chromatography (Partition Chromatography)
Partition Chromatography was introduced by Archer Martin and Richard Laurence. Partition Chromatography is used to separate the component from liquid-liquid phases. In Partition Chromatography, the retention factor is given by the distance travelled by the component from the mobile phase to the stationary phase and then back to the mobile phase. In Partition Chromatography one phase is polar and the other is non-polar. The difference in polarity leads to the separation of components.

A continuous differential partitioning of mixture components into a stationary phase and a mobile phase occurs in this process. Paper chromatography is a good example of partition chromatography. The stationary phase in this procedure is chromatography paper, which is suspended in a combination of solvents acting as the mobile phase.
The mixture to be separated is placed at an area at the bottom of the chromatographic paper, and as the solvent rises up the paper, the components are carried to varying degrees depending on how well they adhere to the paper. As a result, the components are divided at various heights.
Gel Filtration Chromatography
One of the types of Partition Chromatography is Gel Filtration Chromatography. Gel Filtration Chromatography is based on the principle that molecules are partitioned between the stationary phase and a mobile phase that consists of a porous matrix whose porosity is of defined nature.
The column of such a matrix has two measurable liquid volumes named external and internal volumes. The molecules greater than the size of pores of the stationary phase will exclude from internal volume. The molecules whose size is smaller than the pores of the stationary phase make equilibrium with external and internal volume and migrate slowly. These smaller particles exclude the volume larger than the external volume.
Hence, in Gel Filtration Chromatography, the particles are separated in decreasing order of their molecular size. Gel Filtration Chromatography is used to separate proteins and peptides, cells and viruses, and other biomolecules.
Liquid Chromatography
Liquid Chromatography is a chromatographic technique in which the mobile phase is liquid. The solute is dissolved in the liquid mobile phase which is then passed through the column surrounded by the stationary phase or the plane over the stationary phase. The solute dissolved in the liquid phase gets adsorbed to the stationary phase depending on its interaction with the stationary phase. Liquid Chromatography is an essential tool when the mixture that is to be separated is colored. Depending on the components of chromatography Liquid Chromatography is of four types namely, Reversed Phase, Size Exclusion, Normal Phase, and Ion Exchange Chromatography.
Liquid Chromatography is used in testing food quality, forensic labs, testing ink samples, and environmental analysis. It is an easily available, relatively inexpensive technique and performed manually with better control.
Gas Chromatography
Gas Chromatography is a technique used to separate volatile components in the gas phase. Gas Chromatography is a chromatographic technique that uses an inert gas as the mobile phase. When the stationary phase is solid in Gas Chromatography then it is called Gas-Solid Chromatography while when a liquid on an inert layer is used as a stationary phase then it is called Liquid-Gas Chromatography.
A Gas Chromatographer consists of a sample injection unit, a column, and a detector. The sample to be analyzed is injected into the sample injection system which heats up the sample and vaporizes it. After the sample heats up it moves up along with the gas mobile phase through the column which is separated by the stationary phase and the final compound is analyzed by the detector. The technique of Gas Chromatography was introduced by Mikhail Semenovich Tsvett.
Gas Chromatography is extensively used for the analysis of volatile mixtures, in analyzing air samples to analyze air quality, in the pharmaceutical industry, and in analyzing environmental toxins.
Gas-Liquid Chromatography
We know that every Chromatography involves two types of phases named mobile phase and stationary phase. In Gas-Liquid Chromatography, the mobile is a gas like helium and the stationary phase is a liquid which is of high boiling point and is absorbed into a solid. The velocity of movement of a compound in the chromatography machine depends on the time it spent with the gas i.e. the mobile phase.
The working of Gas-Liquid Chromatography can be understood in the following steps:
Step 1: First the sample is injected using a syringe in a temperature-controlled oven. Here, the sample boils and moves into the column in gaseous form with the help of helium gas i.e. mobile phase.
Step 2: The column is packed with porous rock coated with a waxy polymer.
Step 3: The temperature of the column ranges between 50C to 250C. With the proceeding of analysis, the temperature starts rising.
Step 4: Inside the column, the sample may condense, it may dissolve in the liquid, or may remain in the gas. In general, the substance partitions itself between the gaseous mobile phase and the stationary liquid phase. Any molecule of the sample spends some of its time with the liquid stationary phase and some with the gaseous mobile phase.
Applications of Chromatography
Here are some important uses and applications of Chromatography in our everyday life:
Used to Separate the Colors of a Dye
A thin strip of filter paper is taken and a line using a pencil is drawn at a noticeably visible length from the lower corner of the paper. A drop of ink using a fountain pen is projected at the centre of the drawn line. It is dried further. The filter paper is dipped in a beaker containing water as the solvent, in such a way that the ink drop is right above the water level. When the water rises up on the filter strip, it takes along with it the dye particles. Since the dye is soluble in water, the component in the dye with a greater solubility rises higher. As a result of this, the components of the dye get separated.
Chromatography in Food Department
Chromatography can be used to estimate the shelf life of food items by providing insight into when the food item gets spoiled. It can be used to understand the nutritional value of the food sample. It can be also used to analyze the presence of chemical additives in food. Can be used for the clarification and skimming of milk. Extraction of cream and production of cheese.
Chromatography in Chemical Industry
The purity detection of water samples and air samples is all done by Chromatography. Detection of the presence of toxic contaminants in pesticides and oils. The most commonly used technique is GC and HPLC.
Chromatography in Pharmaceutical Industry
Chromatography can be used to detect the presence of chemicals and elements in a given sample, present in traces. These chemical compounds can also be segregated and extracted on the basis of their molecular masses and constituent elements. Separation of drugs from the bloodstream. The removal of impurities and unknown compounds from the drugs as well as the estimation of drug sample purity can be analyzed using the chromatography technique.
Chromatography in Molecular Biology
Nucleic acid research can be simulated by the chromatography technique. EC-LC-MS, an extensive chromatography technique can be used to perform the study of proteomics and metabolomics. HPLC chromatography technique can be used to carry out protein separation operations. Several procedures like plasma fractionation and enzyme purification are based on chromatography.
Read More
FAQs on Chromatography
1. What is Chromatography?
Chromatography is a technique used to affect the separation of two or more dissolved solids contained within a solution in very small quantities. In Greek, the word ‘chroma’ means colour and ‘graphein’ is used to indicate writing. Initially, the technique was used for the separation of colors.
2. What do you mean by Rf value in Chromatography?
Retention factor (Rf) refers to how far a fluid substance goes up a chromatography plate in paper chromatography. All chemicals have a standard RF value for each specific solvent, and RF values are used to compare unknown samples to known compounds.
3. What are Some Applications of Chromatography?
Following are some important applications of Chromatography:
- Paper Chromatography is used to Separate Colors of a Dye.
- Chromatography can be used to estimate the shelf life of food items by providing insight into when the food item gets spoiled.
- Chromatography can be used to detect the presence of chemicals and elements in a given sample, present in traces.
- The purity detection of water samples, and air samples are all done by Chromatography.
4. Explain the Technique used in Chromatography.
In case the substances to be segregated are colorless, then during the process of chromatography, the components may not be visible on the chromatogram. Therefore, the developing technique is the process of spraying a suitable reagent on the chromatogram such that the different components become visible on the chromatogram. The reagent is known as the developing reagent.
5. List down some Limitations of Paper Chromatography.
Paper chromatography has some limitations such as:
- Greater components’ concentration. This may lead to streaking.
- It is semi-quantitative in nature.
- Irregular sample spotting
- Overlap in the spots with close Rf value components.
- Uneven flow of solvent may lead to error in the calculations of Rf values.
6. What is the difference between TLC and Paper Chromatography?
In TLC, Glass Plate coated with Silica Gel is used while in Paper Chromatography the stationary phase is Cellulose filter Paper filled with water in its pores.
7. What Chromatography is Used For?
Chromatography is a separation technique used for separation, purification, and identification of components for qualitative and quantitative analysis.
8. Who discovered Chromatography?
Chromatography was discovered by Mikhail Tsevet
9. How many types of Chromatography?
Chromatography is of many types such as Gas Chromatography, Liquid Chromatography, Adsorption Chromatography, Thin Layer Chromatography, Partition Chromatography, etc.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...