Electric Battery
Last Updated :
02 Nov, 2023
In electrical circuits, electric batteries play an important role in providing the required power to different range of devices and systems. Electric Battery provides the necessary power for small gadgets to large-scale industrial machines. An Electric battery acts as a source of electric power which generally consists of one or more electrochemical cells.
When an electric battery is used for supplying the power, its positive terminal acts as a cathode, and its negative terminal acts as an anode. Electric battery is used in many applications like smartphones, electrical vehicles, and many more applications. Electric batteries are used to provide portable energy for many applications. In this article, we will learn about electric batteries, and key terminologies, properties, working of batteries, electric battery types, advantages and disadvantages, and their applications.
Electric Battery
An electric battery is a source that stores electrical power energy in chemical form and it releases electrical power when required. It consists of one or more electrochemical cells. An electric battery is used for supplying the power, then its positive terminal acts as a cathode, and its negative terminal acts as an anode.

Electric Battery
Key Terminologies of Battery
Batteries are mainly classified into two types:
Primary batteries
Primary batteries are generally designed for one-time used energy sources. These are used until their energy is exhausted and then these are discarded. In primary batteries chemical reactions are not reversible, so that they cannot be recharged again. Once their energy is exhausted, then the battery stops working and it becomes useless.
Secondary batteries
Secondary batteries are generally designed in usage of multiple times. Secondary batteries can be reused multiple times. These can be recharged after completion of their energy source. In Secondary batteries chemical reactions are reversible, so that they can be recharged again and used again.
Properties of Electric Battery
Some properties of Electric Battery are listed below:
- It consists of one or more electrochemical cells which converts chemical energy into electrical energy.
- In each battery cell it has a positive (cathode) terminal and a negative (anode) terminal.
- It acts an voltage source ,which provides an EMF(electromotive force) that helps to drive the flow of current in electrical circuit.
- If an Electric Battery is connected to external power electrical device, then electrons flow from the battery’s negative terminal to battery’s positive terminal, which creates electric current.
- Electric Battery capacity is generally measured in milliampere-hours(mAh) and it shows how much charge a battery can store.
- If the capacity of an Electric is Higher then the batteries are ideal for long-lasting power in devices.
- There are different types of battery like lithium-ion, Lead-Acid Batteries based on their uses.
- Electric Battery consists of Self-Discharge Rate which is crucial for devices like remote controls with infrequent use.

Properties of Electric Battery
Working Principle of Electric Battery
Electric battery is a source that stores electrical power energy in chemical form and it releases electrical power when we required. Electric battery consists of one or more electrochemical cells. An electric battery is used for supplying the power, then its positive terminal acts as cathode and its negative terminal acts as an anode.
Each Electric battery consists of three essential components in it:
- An anode which acts an electrode.
- An Cathode which acts another electrode.
- An Electrolyte.
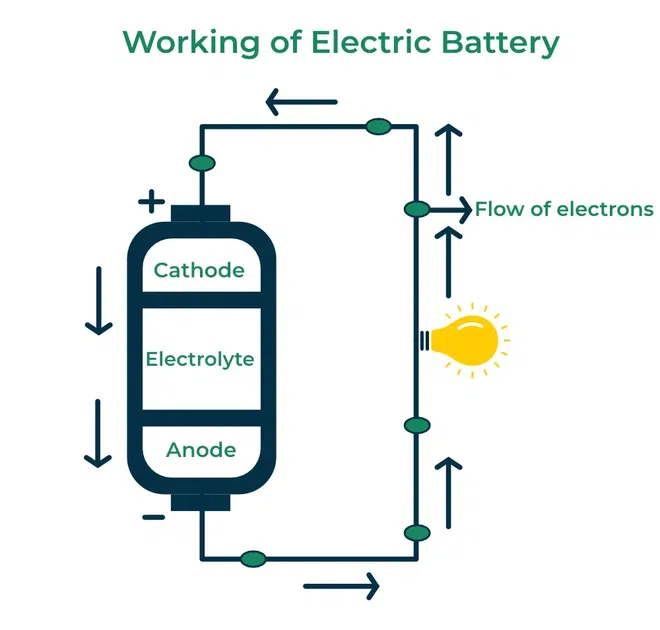
Working of Electric Batteries
When a Electric battery is connected to electrical circuit, then an chemical reaction occurs within its cell. In Chemical reaction ,Near An anode (negative terminal), a chemical reaction takes place where it releases electrons, creating a surplus of electrons at this terminal. Same as, at the cathode (positive terminal), another chemical reaction consumes these electrons. These released Electrons naturally flows from the anode terminal to the cathode terminal through an external electrical circuit. This flow of electrons constitutes an electric current, which can be useful to perform the work in the connected devices.
Types of Electric Battery
There are different types of electric battery:
- Lead-Acid Battery.
- Lithium-Ion Battery.
- Nickel-Cadmium Battery.
- Nickel-Metal Hydride Battery.
Lead-Acid Battery
Lead-Acid Battery is a type of secondary battery. Lead-Acid Battery can be rechargeable multiple times and these are widely used in many applications. Lead-Acid Battery are popularly known for their durability which is mainly used in heavy industrial applications.
- Lead-Acid Battery is mainly known for its reliability.
- Lead-Acid battery continue to be cost-effective choice for many automobile applications.
- Lead-Acid battery is mainly used for auxiliary functions in electric vehicles.
- Lead-Acid battery provides backup power in UPS systems
- Lead-Acid battery are mostly used in automobiles like starter batteries for starting the engine.
- Lead-Acid battery provides power to vehicle’s electrical system when the car engine is off.
Lead-Acid Battery
Lithium-Ion Battery
Lithium-ion (Li-ion) Battery is a type of secondary battery. Lithium-Ion Battery can be used for recharging multiple times and widely used type of rechargeable battery. Lithium-ion (Li-ion) battery is popularly known for their high density, lightweight design and its long-lasting power.
- Lithium-Ion Battery are mainly used in Portable Electronics like smartphones, tablets, laptops etc.
- Lithium-Ion Battery provide high energy density and available in compact size, and it is able to provide long-lasting power.
- Lithium-Ion Battery is used in Electric Vehicles like electric cars, scooters, and bikes.
- Lithium-Ion Battery provides high energy capacity and helps for clean and sustainable environment ,and allows for extended travel distance.
- Lithium-Ion Battery is used in Electronics Accessories like wireless headphones, Bluetooth speakers.

Lithium-Ion Battery
Nickel Cadmium Battery(NiCad )
Nickel Cadmium Battery(NiCad ) is a type of secondary batteries .Nickel Cadmium Battery is popularly known as NiCad battery. Nickel-Cadmium Battery provides an convenient and long-lasting power source for many devices. It is also a type of rechargeable battery.
- Nickel-Cadmium Battery are mainly used in film cameras and Flashes.
- Nickel-Cadmium Battery can have long life cycle.
- Nickel-Cadmium Battery can charge quickly, so that it can be used for rapid charging devices.
- Nickel-Cadmium Battery can be used in wide Temperature Range.
- Nickel-Cadmium Battery used in Portable Power Tools like powered drills.
- Nickel-Cadmium Battery can be used in Emergency Lighting source.

Nickel Cadmium Battery
Nickel-Metal Hydride Battery
Nickel-Metal Hydride Battery is a type of secondary battery. Nickel-Metal Hydride Battery are also known as (NiMH) battery. Compared to nickel-cadmium (NiCd) battery , Nickel-Metal Hydride Battery provide improved capacity, rechargeability, more energy density.
- Nickel-Metal Hydride Battery are mainly used in Electronics devices like digital cameras.
- Nickel-Metal Hydride Battery are used in portable devices like audio players and remote control devices.
- Nickel-Metal Hydride Battery are used in phone handsets, which provides a reliable and rechargeable power source.
- Nickel-Metal Hydride Battery are used in gaming Controllers like play stations.
- Nickel-Metal Hydride Battery are suitable for broad range of electronics.

Nickel-Metal Hydride Battery
Applications of Electric Battery
- Electric Battery like lithium-ion battery used in smartphones.
- Electric Battery like lead-acid batteries in automobiles like car engine and power accessories.
- Electric Battery is used in portable devices like power banks .
- Electric Battery provides source of energy for portable devices like mobile devices.
Advantages of Electric Battery
- Electric Batteries are portable and these can easily supply power to devices without any supply of external power source.
- Electric Batteries have generally longer lifespan and these can store energy for extended periods.
- Electric Batteries have very low maintenance power supply sources compared to other energy storage devices.
- Electric Batteries provide High specific energy, long storage times and instant readiness give primary batteries a unique advantage over other power sources.
Disadvantages of Electric Battery
- Electric Battery has very limited energy storage capacity as compared to other power storage sources.
- Some Electric Batteries have toxic materials, and if we do not dispose them properly it can harm environment.
- Charging the Electric Batteries is an time consuming process, especially when the battery has high-capacity storage.
- Some Electric Batteries have Limited Cycle Life, so they cannot work for longer period of time.
Conclusion
Electric batteries In an electrical circuit play an important role in providing the required power to different range of devices and systems. Electric Battery provides necessary power from small gadgets to large scale industrial machines. An Electric battery acts as an source of electric power which generally consists of one or more electrochemical cells. An electric battery is used for supplying the power, then its positive terminal acts as cathode and its negative terminal acts as an anode. Electric batteries enables flexibility and portability to many electrical devices. Electric batteries have many applications which will be useful in our daily life. We have covered all the applications of Electric batteries. We have learned about the advantages and disadvantages of Electric batteries. Electric Battery consists of Self-Discharge Rate which is crucial for devices like remote controls with infrequent use. There exist many different types of battery like lithium-ion, Lead-Acid Batteries based on their uses.
FAQs : Electric Battery
1. How can we maximize the lifespan of rechargeable battery?
Yes, we can maximize the lifespan of your rechargeable battery by store it in a cool place and avoid deep discharging. You can keep charging them before they deplete completely.
2. Why do we need to recycle old batteries?
Recycling old batteries helps to reduce risks. Recycling old batteries helps in preventing environmental harm chemicals and protects public health. Many batteries has dangerous materials so that, if it is not disposed properly, it can cause harm to our environment and people.
3. Is there any eco-friendly batteries available?
Yes, there any eco-friendly batteries available with lowered environmental impact. Lithium-ion batteries are eco-friendly batteries.
4. It is advisable to use different brands batteries together in a device?
No ,It is advisable to same branded, same type, and same capacity batteries in a device to ensure better and consistent performance.
5. How can we dispose used old batteries safely ?
It generally depends on the battery type. For example Alkaline batteries can usually dispose in your regular trash. And For rechargeable batteries it is advisable to recycle them. You can find local collection centers or drop-off locations. You need to Make sure to tape the terminals and place them in a non-conductive bag to prevent short circuits.
Share your thoughts in the comments
Please Login to comment...