What is Battery and its Types?
Last Updated :
11 Jan, 2024
A battery is a device that generates electric power from the controlled flow of ions(positive and negative ions) which are called chemical reactions or redox reactions later they can be used for a wide range of applications from charging smartwatches to renewable energy to electric vehicles. A battery consists of one or more electrochemical cells with cathode, anode, and electrolyte components. A battery is the best source of electric power which consists of one or more electrochemical cells with external connections for powering electrical devices.
1. Cathode: The cathode is a positively charged electrode. During a chemical reaction, it gains electrons, which is called reduction.
2. Anode: Anodes are negatively charged electrodes. During a chemical reaction, it loses electrons, which is called an oxidation reaction.
3. Electrolyte components: Electrolyte components are generally chemical substances that are used to enhance or allow the flow of ions from the cathode to the anode and anode to the cathode.
Take an example of The zinc and copper redox equation : Zn + Cu2+ → Zn2+ + Cu,
Cathode reaction : Cu2+ + 2e– ⇔ Cu
Anode reaction : Zn ⇔ Zn2+ + 2e–
CuSo4 is used as electrolyte components.
Examples of Battery
There are some important list of examples of batteries given below :
- Lead-Acid Battery
- Nickel-Cadmium Battery
- Lithium-Ion Battery
1. Lead-Acid Battery
It is best known for one of the earliest rechargeable batteries and we can use it as an emergency power backup. It is popular due to its inexpensive facility.
2. Nickel-Cadmium Battery
It is also known as NiCad Battery. It is found in certain toys and small electronic items or gadgets.
3. Lithium-Ion Battery
It is valuable due to its most stable and safe feature. It is having very high energy capacity. It is used in mobiles, laptops , etc..
Characteristics of Battery
- Voltage: Batteries have a specific voltage, which is basically the potential difference between cathode and anode terminal. It’s the force that drives the flow of electrons through a circuit and It determines the electrical potential energy that the battery can produce.
- Capacity: Capacity of a battery represents the amount of electrical charge a it can store and deliver. Also it determines how long a battery can power a device. Unit of capacitance is ampere-hours (Ah) and milliampere-hours (mAh) for small battery.
- Energy Density: Energy density defines the amount of energy a battery can store in per unit of volume or weight. Higher energy density means more energy in a smaller or lighter package. This characteristic indicates how quickly a battery can release its stored energy. Some batteries can provide high current output for short bursts, while others are designed for slow, continuous discharge.
Working of Battery
A battery is a electronics device that generates electric energy from chemical reaction where two electrodes involves as a main part of reaction. One is called anode(negative pole) and the other is called as cathode(positive pole)and they are separated by an electrolyte chemical component. When an external circuit is connected to the cathode and anode, electron starts to flow from the anode to the cathode and creates an electric current. Simultaneously, ions withing the electrolyte migrate between the electrodes to maintain charge balance. These electron and ion exchanges include chemical reactions at both the anode and cathode, resulting in the generation of electrical energy suitable for powering external devices. Over time, as the chemical reactants become depleted, the battery’s capacity decreases, and it needs to recharging or replacement.
Types of Battery
There are various types of batteries. Based on charging capacity we can divide them in two types:
- Primary cell battery
- Secondary cell battery

Primary and Secondary cell battery
1. Primary Cell Battery
Primary cell batteries are designed to be used for once, and discharged. We cannot recharge this type of batteries. Some example of primary cell batteries are.
- Alkaline cells: Alkaline cell is a type of primary cell battery where electrolyte has a PH level of above 7 and mainly potassium or sodium hydroxide is used as electrolyte. zinc and manganese dioxide is used as cathode and anode electrode.
- Aluminium–air battery: Aluminium–air battery is a type of disposable primary battery which produces electric power from the reaction between aluminium and the oxygen in the air. That’s why it is called as aluminium-air battery.
- Atomic battery: Atomic battery or nuclear battery or radioisotope battery that generates electricity from the decay of radioactive isotope. Just like nuclear reaction they produce electric power from nuclear energy. Henry Moseley invented this type of primary battery in 1913. This is generally used in spacecraft, pacemaker etc.
- Daniel cell or Galvanic cell: Daniel cell consists two electrodes generally Cu and Zn, and react electrode is in contact of it’s own ionic solution. For Cu the solution will be CuSo4 and For Zn it is ZnSo4.
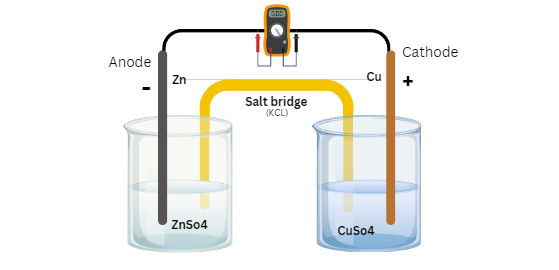
Daniel cell
These are the main types of primary cell battery. Their are some other types such as lead-acid cells, Ni-Cd batteries, Ni-MH batteries, and LI-Po batteries. But mostly used batteries are described above.
Applications of Primary Battery
- Portable Electronics: Primary batteries are widely used in portable electronics devices such as portable camera, calculator, watch, remote smoke detector etc.
- Medical equipment: Their are such medical instruments where primary batteries are used as power source for their long term service. Hearing aids, glucose monitor, weight machine are some example those equipments.
- Toys: There are several toys for children and adult as well where we see the use of primary non chargeable batteries. It can provide long term service and provides power for extended play.
- Emergency backup: There are lot of instruments, where generally secondary batteries are used, but for safety purpose on emergency time, this type of primary non chargeable single use batteries are given more priority for backup.
- Space Mission: In major space mission we don’t have charging system, that’s why primary batteries are used. Also, it gives more power without charging for extended time period.
Advantages of Primary Batteries
- Ready to use : Primary batteries are for useful as they don’t need to charge after buying, You directly use it.
- Long shelf life : As it can’t recharge itself, that’s why it made in such a way so that it can provide more shelf time as compared to others.
- High initial voltage : Initially the new battery provides a stable and high voltage due to its fast force chemical reaction which makes it perfect use for devices that require consistent power.
- Wide temperature range : This characteristics of primary battery makes them perfect for outdoor use in extrema environment.
- Low self-discharge : primary battery retain their energy for long time use.
Disadvantages of Primary Batteries
- Non-rechargeable: Primary battery are one time use only, once it discharged, there are no use of it. For a machine, where it need to supply power for long time, we cannot use primary battery. It will make more expensive in long run.
- Limited capacity: As it is non rechargeable, and limited use only, so only it depleted, there is no of it.
- Environmental impact (waste): Once a primary battery depleted. disposal of such type of batteries can lead to pollution of our environment because of heavy metals like mercury, cadmium.
- Potential cost over time: We need to change the battery over time when it gets depleted. Their is no way to plate it again. So continuously it charges maintenance and a initial cost.
- Performance declines with use: Over time, primary battery declines it’s performance.
2. Secondary Cell Battery
Secondary cell batteries are those types of battery which can be recharged after once it get discharged. Examples of some secondary cell batteries are :
- Nickel-Cadmium Batteries (NiCd): Nickel Cadmium batteries are type of rechargeable battery which use nickel oxide hydroxide and metallic cadmium as electrodes. It has the cycle durability of 2000 cycles and nominal cell voltage is 1.2 V.
- Lead Acid: Lead Acid battery is an example of rechargeable battery which use lead(PbSo4) as cathode anode electrode and sulfuric acid as electrolyte. This is invented in 1859 French physicist Gaston Planté. It has a nominal cell voltage of 2.1 V.
- Nickel-Iron Batteries (Ni-Fe): This rechargeable battery use nickel(III) oxide-hydroxide as positive electrode and iron as negative electrode in presence of potassium hydroxide as electrolyte. This type of batteries are generally used for railroad signaling, trucks/forklifts and mines. It has a nominal cell voltage of 1.2 V.
- Sodium-Ion Batteries: This type of battery use Sodium(Na) as their charge carrier ion.
- Lithium ion: Lithium ion battery is a type of rechargeable battery which gets charged and discharged by lithium ion movement between positive electrode and negative electrode. It generally uses reversible reduction of lithium ions to store energy. It have a nominal cell voltage 3.7V per cell.
.png)
lithium ion battery
Application of Secondary Cell Battery
Secondary cell batteries are widely used due to it’s ability to be charged and multiple time use capacity.
- Electronics devices: Secondary cell batteries are used in maximum electronics devices such as mobile phone, laptop, headphones, tablet, charging light etc.
- Transportation: Currently secondary batteries are used in transportation system in Electric vehicles, Hybrid electric vehicles (HEVs), Electric bikes and scooters.
- Renewable Energy Storage: Storing excess energy generated from solar panels and wind turbines for later use.
Except these use we can see secondary cell battery or rechargeable batteries in Aerospace and Aviation, Military and Defense, Material Handling, Home Energy Storage etc.
Advantages of Secondary Batteries
- Reusable and rechargeable: It can be used for multiple time in long term by recharging.
- Cost-effective in the long run: As it don’t need to change the whole battery, so it’s more cost effective.
- Reduced environmental impact: It less harmful for the environment as compared to primary battery also it don’t need a frequent disposal phase.
- Higher capacity: Secondary battery provides higher capacity as compared to primary battery.
- Convenient for frequent use: It can be recharged and used for multiple time frequently.
- Versatility: Suitable for a wide range of applications.
Disadvantages of Secondary Batteries
- Require charging time: It can be charged, this type of battery. It has to wait and pause the working of machines during the charging period which are connected to the battery.
- Limited lifespan (finite charge cycles): Although it can be charged, it’s not for lifetime use. It also have a limited time of charging and discharging capacity.
- Initial purchase cost: It initially cost higher than a primary battery.
- Slightly lower voltage output: The output voltage of a secondary battery is lower that a primary battery.
Difference Between Primary Cell and Secondary Cell Battery
| Rechargeability |
Non-rechargeable: Single-use |
Rechargeable: Can be recharged multiple times |
| Typical Chemistry |
Examples include zinc-carbon, alkaline, lithium, etc. |
Examples include lithium-ion, nickel-metal hydride (NiMH), lead-acid, etc. |
| Voltage |
Fixed voltage, usually around 1.5V |
There is no fixed voltage. It varies by type, example : 3.6V for Li-ion, 1.2V for NiMH |
| Capacity |
Lower capacity compared to rechargeable |
Higher capacity, suitable for high-drain devices |
| Initial Cost |
Generally more affordable |
initial cost is high |
| Maintenance |
No maintenance needed |
Proper charging and maintenance needed |
For more detail you can refer to – Difference Between Primary Cell and Secondary Cell Battery
FAQs on Battery
1. How do I dispose of primary cell batteries responsibly?
Primary batteries should be recycled at designated recycling centers to prevent environmental contamination.
2. How do I prolong the lifespan of secondary cell batteries?
Avoid deep discharges, store at appropriate temperatures, and use a compatible charger to extend the life of secondary cell batteries.
3. Which type of battery is commonly used in everyday electronics?
Primary cell batteries are often used in everyday devices like remote controls, while secondary cell batteries are common in smartphones and laptops.
4. What is the difference between lithium-ion (Li-ion) battery and lithium primary battery?
lithium-ion battery are rechargeable battery and lithium primary battery is non-rechargeable battery
5. What is salt bridge ?
Salt bridge is an electrochemical device used to connect oxidation and reduction half cell of a galvanic cell. It maintains electrical neutrality within the internal circuit.
Share your thoughts in the comments
Please Login to comment...