Difference Between Isothermal and Adiabatic Process
Last Updated :
21 Sep, 2023
Isothermal and Adiabatic Processes are two different processes that are related to heat and energy in thermodynamics. Thermodynamics is the branch of physics which deals with the heat transfer and thermal properties of matter. In thermodynamics, the isothermal process is the process that occurs at constant temperature and the exchange of heat and energy takes place between the system and the surroundings. Adiabatic Process in thermodynamics stands for the process where a system is isolated from its surroundings and there is no heat and energy exchange between the system and the surroundings.
In this article, we will learn briefly about the isothermal and adiabatic processes, with a major focus on the difference between isothermal and adiabatic processes.
What is Isothermal Process?
Isothermal is derived from two words ‘isos’ meaning same or equal and ‘thermos’ meaning temperature or heat. The process in which the temperature of a system remains constant is termed as isothermal process. As the temperature of a system remains constant in an isothermal process, it means that there is an exchange of heat between the system and the surroundings.
According to ideal gas equation PV = nRT where P, V, n, R, and T represent pressure, volume, number of moles, universal gas constant, and temperature respectively. As n and R are constant and in the case of the isothermal process, T also becomes constant, we can say that PV = constant which is the equation of Boyle’s law. Thus an isothermal process follows Boyle’s law.
Read more about Idael Gas Law.
Work Done In an Isothermal Process
To calculate, the work done in an isothermal process, consider a gas that expands at temperature T, P1 and V1 be the initial pressure and temperature of the gas, and P2 and V2 be the final pressure and temperature of the gas. Assume that the volume of gas changes from V1 to V2 at an intermediate pressure P. Thus work done can be given by:
ΔW = PΔV
As ΔV → 0
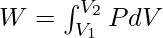
As P = nRT/V from ideal gas equation
![Rendered by QuickLaTeX.com W =nRT\int^{V_2}_{V_1} \frac{dV}{V}\\ W = nRT [\ln (V_2)-\ln(V_1)]](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-4fb973e90871ca1792ef67ddb1daac82_l3.png)
Examples Of Isothermal Process
Some examples of isothermal process are mentioned below:
- Carnot Engine follows isothermal process in which the fluid experiences isothermal compression and expansion.
- Change of phase of a substance also follows isothermal process. For example change of solid ice to liquid water is an isothermal process.
Graph Of Isothermal Process
We know that work done in an isothermal process is given by ![Rendered by QuickLaTeX.com W = nRT [\ln (V_2)-\ln(V_1)]](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-50ab70b73c90317a6b39c6069f355830_l3.png) . The graph of isothermal process is shown below. The area of the graph provides the amount of work done in the isothermal process.
. The graph of isothermal process is shown below. The area of the graph provides the amount of work done in the isothermal process.

What is Adiabatic Process?
The word adiabatic has its origin in the Greek word ‘adiabatos’ meaning impassable. Thus the system is a closed system in case of an adiabatic process. So there is no exchange of heat or matter between the system and surroundings. There are 2 important conditions for an adiabatic process to occur which are:
- The system should be a closed system i.e. it must be completely insulated.
- The process must occur rapidly.
An adiabatic process can be reversible as well as irreversible.
Examples Of Adiabatic Process
Some examples of adiabatic process are as follows:
- Ice kept in an icebox is the example of adiabatic process as no exchange of heat takes place between the surroundings and the system (ice box).
- Process of sound propagation in air is an example of adiabatic process.
Work Done In An Adiabatic Process
In an adiabatic process, the ideal gas equation becomes PVγ = k where P, V, k, and γ represent the pressure, volume, any constant and specific heat at constant pressure and volume respectively. The value of γ is given by:
γ = Cp/Cv
where Cp and Cv are the specific heat at constant pressure and specific heat at constant volume respectively.
We know that
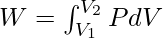
As 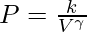
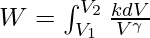
![Rendered by QuickLaTeX.com W = \frac{k}{1-\gamma}[V_2^{1-\gamma}-V_1^{1-\gamma}]](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-a9921fc2108be6517a1864737d2b053a_l3.png) its
its
Graph Of An Adiabatic Process
We know that work done in an adiabatic process is given by ![Rendered by QuickLaTeX.com W = \frac{k}{1-\gamma}[V_2^{1-\gamma}-V_1^{1-\gamma}]](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-8304b7cc6b73eb29785376504b4b0fbb_l3.png) . The graph of adiabatic process is shown below. The area of the graph provides the amount of work done in the adiabatic process.
. The graph of adiabatic process is shown below. The area of the graph provides the amount of work done in the adiabatic process.

Difference Between Isothermal And Adiabatic Process
The differences between isothermal and adiabatic processes are discussed in the table below:
|
| The system in an isothermal process is an open system. | The system in an adiabatic process is a closed or insulated system. |
| Heat or energy transfer takes place between the system and its surroundings. | No heat transfer or exchange of energy takes place between the system and its surroundings. |
| The pressure in the system at a given volume is more. | The pressure at a given volume is less. |
| The isothermal process is a slow process. | Adiabatic process is very quick. |
| Change of phase of a substance is an example of an isothermal process. For example, the change of solid ice to liquid water is an isothermal process. | Process of sound propagation in air is an example of an adiabatic process. |
Also, Check
FAQs on Isothermal and Adiabatic Processes
1. Define Isothermal Process.
The process in which the temperature remains constant is called an isothermal process.
2. Define Adiabatic Process.
A process in which there is no exchange of heat or energy or matter between the system and the surroundings is known as an adiabatic process.
3. Give an Example of Isothermal Process.
Carnot Engine follows isothermal process in which the fluid experiences isothermal compression and expansion.
4. What is an Example of Adiabatic Processes?
Process of sound propagation in air is an example of adiabatic process.
5. Write the Equation to Calculate the Work Done in an Isothermal Process.
Work done in an isothermal process is calculated using
![Rendered by QuickLaTeX.com W = nRT [\ln (V_2)-\ln(V_1)]](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-2925cfcbe26ee4544f1abe72e4159666_l3.png)
6. Write the Equation to calculate the Work Done in an Adiabatic Process.
Work done in an adiabatic process is calculated using
![Rendered by QuickLaTeX.com W = \frac{k}{1-\gamma}[V_2^{1-\gamma}-V_1^{1-\gamma}]](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-a9921fc2108be6517a1864737d2b053a_l3.png)
7. Distinguish between Isothermal and Adiabatic Processes.
Main difference between an isothermal and an adiabatic process is that in an isothermal process heat transfer takes place between the system and the surroundings while there is no heat transfer in an adiabatic process.
8. What does γ Represent in an Adiabatic Process?
γ in an adiabatic process represent the ratio of specific heat capacities at constant pressure and constant volume.
Share your thoughts in the comments
Please Login to comment...