Zero Order Reaction is a type of chemical reaction where the reaction rate does not change with variations in the concentration of the reactants. In such reactions, the concentration rates remain constant over time. This type of reaction is characterized by the fact that the concentration of the reactants does not influence the reaction rate.
In this article, we look into What the order of the reaction is, what the zero-order reaction is, definition characteristics, rate law expression, etc.
What is Order of Reaction?
Order of a reaction refers to the relationship between the rate of a chemical reaction and the concentration of the species involved. It can be determined experimentally by observing how reactant concentration changes affect the reaction rate.
The overall order of a reaction is calculated by summing the individual orders of the reactants involved. Thus, based on order, reaction can be:
- Zeroth Order Reaction
- First Order Reaction
- Second Order Reaction
Note: The order of a reaction can even be fractional as well.
In this article, we will discuss the zeroth-order reaction in detail.
What is a Zero Order Reaction?
A zero-order reaction is a type of chemical reaction where the rate of the reaction remains constant and is independent of the concentration of the reacting substances. In a zero-order reaction, the concentration of the reactants does not change over time, and the reaction rate is always equal to the rate constant of the specific reaction. This means that increasing or decreasing the concentration of the reactants does not affect the reaction rate. The rate equation for a zero-order reaction is simply the rate constant multiplied by time.
Zero Order Reaction Definition
A zero-order reaction is a type of chemical reaction where the rate of the reaction remains constant and is independent of the concentration of the reacting substances.
Characteristics of Zero Order Reactions
Zero Order Reaction demonstrate the following characteristics:
- The rate of the reaction is not proportional to the reactant’s concentration.
- The reaction rate is related to the square of the reactant’s concentration.
- The natural logarithm of the reactant’s concentration determines the reaction rate, showing a proportional relationship.
- Situations leading to zero-order rates include a small fraction of reactant molecules being in a reactive state, constantly replenished, or when reactants have significantly different concentrations.
- Zero-order reactions are sometimes called pseudo-zero-order reactions due to being artifacts of specific reaction conditions.
Examples of Zero Order Reactions
Some Examples of zero-order reactions are:
- Reaction of Hydrogen with Chlorine (Photochemical Reaction)
[Tex]\text{H}_2(g) + \text{Cl}_2(g) \xrightarrow{\text{light}} 2\text{HCl}(g) [/Tex]
- Decomposition of Nitrous Oxide over a Hot Platinum Surface
[Tex]2 \text{N}_2\text{O}(g) \rightarrow 2 \text{N}_2(g) + \text{O}_2(g)[/Tex]
- Iodization of Acetone (In H+ Ion Rich Medium)
[Tex]\text{CH}_3\text{COCH}_3 + \text{I}_2 + \text{H}^+ \rightarrow \text{CH}_3\text{COCH}_2\text{I} + \text{H}_2\text{O}[/Tex]
- Constant Breakdown of Alcohol in the Liver
Rate Law for Zero Order Reactions
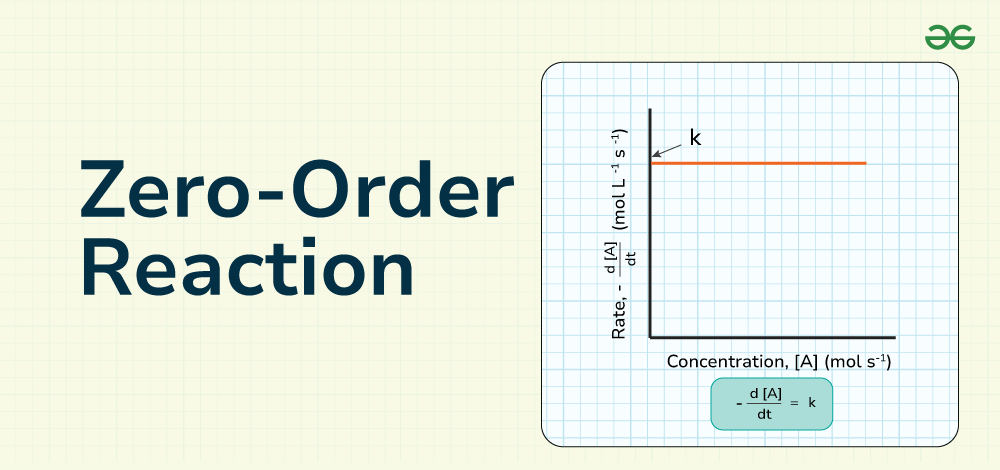
In a zero-order reaction, the rate is independent of the concentration of the reactants, and the rate constant directly determines the rate of the reaction. Rate law of a zero-order reaction is defined by the expression rate = k, where “rate” represents the reaction rate and “k” is the rate constant.
In a zero-order reaction, the rate is independent of the concentration of the reactants, and the rate constant directly determines the rate of the reaction. This means that changing the concentration of the reactants does not impact the rate of the reaction, making the rate constant the sole factor influencing the reaction rate in a zero-order reaction.
Formula for Zero Order Reaction
For reaction A → B, rate is given as:
Rate = k = – Δ[A]/Δ𝑡
Where,
- k is rate constant, and
- Δ[A]/Δ𝑡 is rate change of concentration of reactant.
Unit of Rate Constant for Zero Order Reaction
As for zero order reaction, rate is same as rate constant as rate doesn’t depends on the concentration of reactants. Thus, unit of rate or rate constant is same.
Unit of rate constant for zero order reaction is 1/s or 1/min or 1/hr.
Read More about Integrated Rate Laws.
Factors Affecting Rate of Zero Order Reactions
Factors that affect zero-order reactions are :
- Concentration of Reactants: In zero-order reactions, the rate remains constant regardless of changes in reactant concentrations, making it independent of reactant amounts.
- Rate Constant: The rate law for a zero-order reaction is rate = k, where k is the rate constant expressed in concentration/time units like M/s.
- Half-Life: The half-life of a zero-order reaction depends on the rate constant and the initial concentration of the reactant, as shown in the equation t1/2 = [A]0 / 2k.
Read More, Factors Affecting Rate of a Chemical Reaction.
Half-Life Time (t1/2) for Zero Order Reaction
For a zero-order reaction, the half-life (t1/2) is the time required for the concentration of the reactant to decrease to half of its initial value. The half-life for a zero-order reaction can be calculated using the following formula:
It is the time for half conversion of reactants into products.
t½ = [A]o/2k
Where,
- t1/2 is the half-life of the reaction,
- [A]o is the initial concentration of the reactant, and
- k is the rate constant of the zero-order reaction.
Differential and Integrated form of Zero Order Reaction
In the differential form, the rate of change of the concentration of the reactant with respect to time (d[A]/dt) is equal to the negative of the rate constant (−k) multiplied by the concentration of the reactant ([A]). This can be expressed as:
d[A]/dt = -k
To derive the integrated form of the zero-order reaction, we can integrate the differential rate equation. Assuming the initial concentration of the reactant ([A]o) is known, the integrated form is:
[A] = [A]o – kt
Where:
- [A] is the concentration of the reactant at time t,
- [A]o is the initial concentration of the reactant,
- k is the rate constant of the zero-order reaction, and
- t is the reaction time.
Zero, First and Second-Order Reactions
The comparison of zero order, with the first-order and second-order reactions is as follows:
Features
| Zero-Order
| First-Order
| Second-Order
|
|---|
Rate law
| Rate = K
| Rate = k[A]
| Rate = k[A]2 or k[A][B]
|
|---|
Integrated rate law
| [A] = -kt + [A]_0
| ln[A] = -kt + ln[A]_0
| 1/[A] = kt + 1/[A]_0
|
|---|
Graphical behavior
| Concentration vs. time yields a straight line with a negative slope
| ln[Concentration] vs. time yields a straight line
| 1/[Concentration] vs. time yields a straight line
|
|---|
Half life time
| Half-life is directly proportional to the initial concentration
| Constant half-life
| Half-life increases with decreasing concentration
|
|---|
Reaction order
| Zero order with respect to reactant
| First order with respect to reactant
| Second order with respect to reactant
|
|---|
Reaction constant unit
| Molarity per time (M/s)
| Inverse time (s-1)
| M-1 s-1
|
|---|
Example
| Enzyme-catalyzed reactions
| Radioactive decay
| Dimerization of molecules
|
|---|
Experimental data
| Concentration changes linearly over time
| Concentration changes exponentially over time
| Concentration changes quadratically over time
|
|---|
Reaction
| Rate is constant
| Unimolecular Process
| Bimolecular Process
|
|---|
Significance of Zero Order Reactions
The significance of a zero-order reaction lies in its unique characteristics and implications in chemical kinetics. In a zero-order reaction, the reaction rate is independent of the reactant(s) concentration and solely depends on the rate constant (k). This has several important implications:
- Rate Independence: Zero-order reactions are not influenced by changes in reactant concentrations. This can be crucial when maintaining a constant reaction rate regardless of whether reactant levels are desired.
- Steady Reaction Rate: The rate of a zero-order reaction remains constant over time, providing predictability and stability in specific chemical processes.
- Reaction Mechanism Insights: Understanding zero-order reactions can shed light on reaction mechanisms, especially in cases where reactants are limited or catalysis plays a significant role.
- Practical Applications: Zero-order reactions are found in various real-life applications, such as enzyme kinetics, drug metabolism, and specific chemical processes where maintaining a constant rate is essential.
- Experimental Design: Recognizing and studying zero-order reactions is crucial for experimental design, especially when dealing with reactions that exhibit unusual concentration dependencies.
- Kinetic Studies: Zero-order reactions serve as a valuable model for studying reaction kinetics and understanding the relationship between reaction rates and reactant concentrations.
Related Articles
Sample Problems on Zero Order Reactions
Question 1: For a zero-order reaction A →P, if the initial concentration of A is 1.0 M and after 20 seconds, the concentration of A decreases to 0.6 M, calculate the rate constant k.
Solution:
The rate of a zero-order reaction is given by the equation: Rate = -k.
Using the given data, we can calculate the rate constant k as follows:
Rate = (1.0 M – 0.6 M) / 20 s = 0.4 M / 20 s = 0.02 M/s.
Therefore, the rate constant k for this reaction is 0.02 M/s.
Question 2: In a zero-order reaction A → B, if the rate constant k is 0.005 M/s, what will be the concentration of A after 50 seconds if the initial concentration of A is 2.0 M?
Solution:
The concentration of A at any time t in a zero-order reaction is given by the equation:
[A]=[A]0 −kt
Substituting the given values into the equation:
[A]=2.0M−(0.005M/s×50s)=2.0M−0.25M=1.75M.
Therefore, the concentration of A after 50 seconds will be 1.75 M.
Question 3: For a zero-order reaction A →P, if the rate constant k is 0.01 M/s and the initial concentration of A is 3.0 M, calculate the time taken for the concentration of A to decrease to 1.0 M.
Solution:
Using the zero-order reaction equation
[A]=[A]0−kt,
we can rearrange it to find the time t:
t = ([A]0 −[A])/k.
Substituting the given values:
t = (3.0M−1.0M)/0.01M/s = 200s.
Therefore, it will take 200 seconds for the concentration of A to decrease from 3.0 M to 1.0 M in this zero-order reaction.
Practice Questions: Zero Order Reaction
Question 1: Calculate the rate constant for a zero-order reaction if the reactant concentration was 0.070 M after 160 seconds and 0.025 M after 345 seconds.
Question 2: In a zero-order reaction, if the initial concentration of the reactant is 0.10 M and it decreases to 0.075 M, with a rate constant of 0.0030 mol L–1 s–1, how long will it take for this change to occur?
Question 3: A zero-order reaction has a rate constant of 0.005 M/s. If the initial concentration of the reactant is 2.0 M, calculate the concentration of the reactant after 50 seconds.
Question 4: For a zero-order reaction A →P, if the rate constant k is 0.01 M/s and the initial concentration of A is 3.0 M, determine the time taken for the concentration of A to decrease to 1.0 M.
Question 5: If a zero-order reaction has a rate constant of 0.0025 M/s, and the initial concentration of the reactant is 1.5 M, calculate the time required for the concentration of the reactant to decrease to 0.8 M.
Zero Order Reaction: FAQs
What is a Zero Order Reaction?
A zero-order reaction is a chemical reaction where the rate remains constant regardless of changes in reactant concentrations. The rate of these reactions is always equal to the rate constant of the specific reactions.
What are the Characteristics of Zero Order Reaction?
The rate of reaction is independent of the reactant’s concentration. The reaction rate is related to the square root, square, or natural logarithm of the reactant’s concentration. Zero-order reactions can occur when only a small fraction of reactant molecules are in a reactive state or when some reactants have significantly higher concentrations.
How is the Rate Law Defined for a Zero Order Reaction?
In a zero-order reaction, the rate law is expressed as rate = k, where k is the rate constant. The rate constant for a zero-order reaction is typically expressed in concentration/time units like M/s.
What Determines the Half-Life of a Zero Order Reaction?
The half-life of a zero-order reaction depends on the rate constant and the initial concentration of the reactant. It can be calculated using the formula:
t1/2=[A]0/2k
How Can You Identify a Zero Order Reaction?
A zero-order reaction can be identified by observing that changes in reactant concentrations do not affect the reaction rate. If increasing or decreasing the concentration of reactants has no impact on the reaction rate, it indicates zero-order kinetics.
Share your thoughts in the comments
Please Login to comment...