Esterification is the chemical process in which alcohol (ROH) reacts with specific acids, predominantly carboxylic acid (RCOOH), to form an ester (RCOOR). An ester is a chemical compound which is having two carbon groups bonded to a single oxygen atom.
In this article, we are going to learn about the process of esterification, exploring its mechanism, techniques, applications, conditions, and examples, along with common frequently asked questions (FAQs).
What is Esterification?
Esterification can be defined as a chemical reaction that forms at least one ester product. It can also be understood as the process of combining an organic acid with alcohol to form an ester and water. Here, alcohol and carboxylic acid undergo an esterification reaction that forms the ester.
The chemical reaction for esterification can be understood by the image given below:

Esterification Reaction
An ester is formed when primary alcohol and carboxylic acid are heated together with the assistance of an acid catalyst. The acid catalyst used in the process is usually sulphuric acid and in other cases, dry hydrogen gas can be used. The compound that is obtained here is known as an ester. The esterification reaction is the name given to the chemical process that results in the formation of the ester.
CH3COOH + CH3CH2COOH → CH3COOCH2CH3
Esterification Mechanism
Esterification proceeds in several steps. The mechanism of esterification is shown below:
Step 1: Formation of cation

Step 2: Here, a delocalized carbocation is formed as a result of protonating the carboxyl oxygen. This, in turn, makes the carbocation a better electrophile.

Step 3: In this step, a proton is then transferred to one of the hydroxyl groups to create a suitable leaving group.

Step 4: Here, the carbon atom forms a π bond by removing water after the alcohol oxygen atom of the hydroxy group delivers a pair of electrons to it. Since the concentration of water is lower than that of methanol, this is not a suitable nucleophile for reversing the reaction.

Step 5: In the final step, the concentration of water is too low to reverse the entire reaction.

Esterification Methods
There are various methods present for preparing esters in laboratories. Some of these methods are discussed below:
Acid Chloride and Alcohol
When acyl chloride reacts with alcohol, its resulting reaction can be quite violent at room temperature. This reaction produces an ester along with hydrogen chloride gas’s acidic fumes.
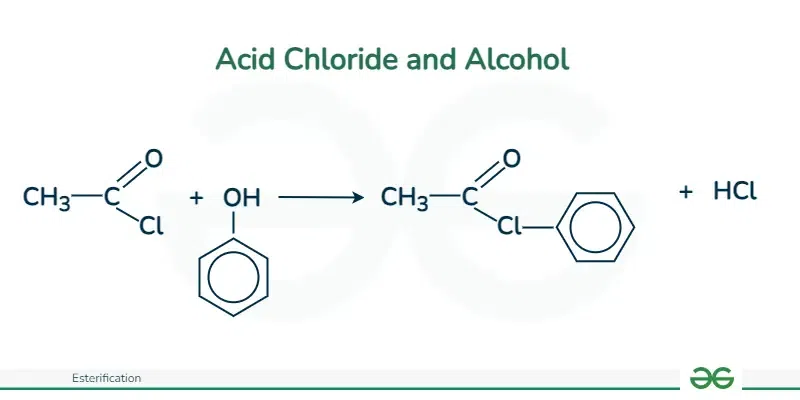
For example, when liquid acetyl chloride is introduced to ethanol, it produces hydrogen chloride fumes which results in forming ethyl ethanoate, which is an ester.
Both phenols and alcohols react when we use this approach. When it comes to phenols, there are situations when changing phenol into a more reactive form beforehand may enhance the reaction.
Acetyl chloride also reacts with phenol in a similar way to the ethanol reaction, but here reaction is comparatively less intense. This reaction produces phenyl ethanoate and hydrogen chloride gas.
Acid Anhydride and Alcohol
The reaction between alcohol and acid anhydride is relatively slower in comparison to the reaction between an acid anhydride and acid chloride. The mixture needed to be warmed to obtain many esters.
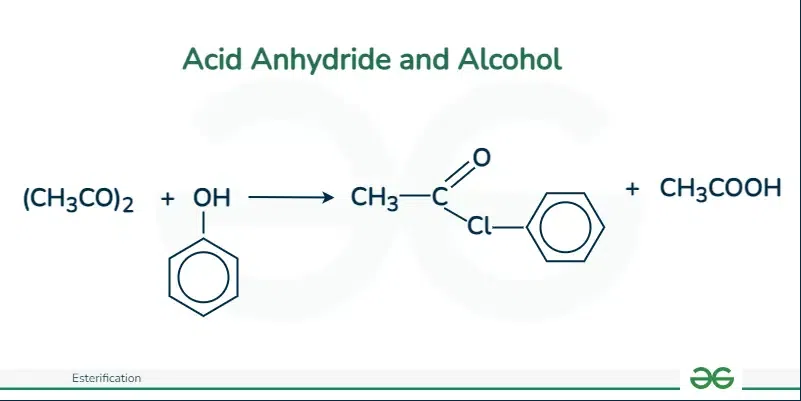
For example, the reaction between ethanol and ethanoic anhydride. Here the reaction is extremely slow at room temperature but speeds up on warming. Even though the colorless liquids seem unchanged, they actually form a mixture of ethyl acetate and acetic acid. A similar reaction takes place with phenol however more slowly.
Carboxylic Acid and Alcohol
This method is used for converting alcohols into esters. Here, esters are formed by heating carboxylic acids and alcohol together with the assistance of an acid catalyst.

The catalyst that is commonly used is concentrated sulfuric acid. Sometimes, dry hydrogen chloride gas is used, but this is usually only for aromatic esters(carboxylic acid containing a benzene ring).
CH3COOH + CH3CH2OH ⇌ CH3COOCH2CH3 + H2O
Here, the ethanol and ethanoic acid react to form water and methyl-ethanoate.
Conditions For Esterification
The conditions that are required for esterification are as follows:
- The presence of a carbonyl compound is a must for forming an ester. Carbonyl compounds such as a carboxylic acid or an acid chloride act as the electrophilic component.
- The process requires carbon electrophile if the carboxylic acid is acting as a nucleophile.
- The process also requires primary alcohol if the carbonyl is acting as the electrophile.
Properties of Esters
Understanding the properties of esters is important. Here are some key properties of esters:
Odor: Esters are known for their pleasant, fruity, and floral odors. They are commonly used in flavorings and perfumes.
Solubility: Esters are generally soluble in organic solvents but they have limited solubility in water due to their non-polar nature.
Boiling Point: The boiling points of esters are typically lower in comparison to the alcohols and acids of similar molecular weight.
Reactivity: Ester reacts when they come into interaction with a base or an acid. This reaction results in forming both the alcohol and the carboxylic acid and is known as saponification.
Flammability: Esters are flammable compounds and they can form explosive mixtures with air.
Uses of Esters
Esters are used in many different ways which are as follows:
- Esters with fragrances are used in food, cosmetics, and perfumes.
- They are used as solvents in paints, inks, and coatings
- They are used in the manufacturing of soaps and detergents.
- They are used as plasticizers in plastics and polymers.
- The formation of the backbone of DNA molecules’ backbone is done by phospho esters.
- They are commonly used as surfactants, oils, and additives.
Examples of Esters
Following are the examples of esters:
- Ethyl acetate: It is found in nail polish remover and fruit flavorings.
- Methyl salicylate: It is used as a flavorful ingredient and found in topical pain relievers.
- Isopropyl myristate: It is often found in cosmetics and personal hygiene items.
- Ethyl butyrate: It adds a fruity flavor to both drinks and foods.
- Glyceryl triacetate: It is used in food and medicine.
- Ethyl formate: It is used to add flavor and make perfumes.
- Isobutyl acetate: It is used as a solvent in paints and coatings.
Difference between Esterification and Saponification
Esterification is the reaction of preparation of ester while saponification is the reaction of preparation of soap. The difference between esterification and saponification is tabulated below:
|
Aspect
|
Esterification
|
Saponification
|
|
Definition
|
Esterification refers to the chemical process that results in the formation of an ester.
|
Saponification refers to the chemical process that results in the formation of soap.
|
|
Reactants
|
Reaction takes place in the presence of alcohol and carboxylic Acid.
|
Reaction takes place in the presence of ester and alkaline (usually a strong base).
|
|
Catalyst
|
Requires acid catalyst.
|
Requires base catalyst.
|
|
Reaction Condition
|
Typically requires heat to proceed, though the conditions can vary.
|
Requires an aqueous alkaline environment and often heat for the reaction.
|
|
Usage
|
Esters with fragrances are used in food, cosmetics, and perfumes.
|
Saponification is used to form soaps that are having various characteristics.
|
|
Reversibility
|
It is a reversible reaction.
|
It is an irreversible reaction.
|
Conclusion
We have learned in this article that esterification is a fundamental chemical process that involves the formation of esters by the reaction between alcohol and carboxylic acid. This process requires acid catalyst. It has various methods and conditions for its execution. Understanding the properties, uses, mechanism, and examples of esters is important for students, So that they can apply it practically in various industries like materials science, food, pharmaceuticals, and cosmetics.
Related Articles
Esterification FAQs
What is the reaction of esterification?
An ester is formed through a process known as an esterification reaction. This reaction usually occurs between an alcohol and either an acid chloride or a carboxylic acid.
What is the purpose of esterification?
Forming ester molecules that have various uses is the purpose of esterification. They can be used in making plastics, medicines, and soaps
What is the chemical equation for esterification?
The esterification chemical equation is given as: RCOOH + R’OH ⇌ RCOOR’ + H2O
Is esterification reversible?
The process of esterification, which involves the reaction between an alcohol and a carboxylic acid, is reversible.
What is the difference between Esterification and Saponification?
Esterification refers to the chemical process in which alcohol reacts with specific acids, predominantly carboxylic acid, to form an ester.
Chemical Equation: CH3COOH + C2H5OH ———> CH3COOC2H5 +H2O
Saponification refers to the process when ester reacts with an acid or a base. This reaction results in forming both the alcohol and the carboxylic acid and is known as saponification.
Chemical Equation: CH3COOC2H5 ———–> CH3COOH + C2H5OH
Give two main uses of the esterification reaction.
The esterification reaction has the following two main applications:
- Manufacturing medicines.
- Manufacturing dyes and paints.
Share your thoughts in the comments
Please Login to comment...