Crystal Structure refers to the arrangement of atoms or molecules in a crystalline material. When atoms or molecules are arranged in a specific repeating pattern, they form a crystal. This arrangement is called the crystal structure. This arrangement gives crystals their unique shapes and properties. Based on the different arrangements, there are seven types of crystals forming crystal system. These crystal systems are further categorized into fourteen different arrangements.
In this article, we will learn in detail about crystal structure, unit cell, lattice structure, seven crystal systems, and more.
What are Crystal Structures?
Crystal structures refer to the specific arrangements of atoms, ions, or molecules within a crystal lattice. In a crystal, these particles are arranged in a regular, repeating pattern, forming a three-dimensional network. This arrangement gives crystals their characteristic shape and properties. Different types of crystal structures exist, such as cubic, tetragonal, orthorhombic, monoclinic, triclinic, hexagonal, and rhombohedral, each with unique symmetries and characteristics.
Crystal structures are like the blueprints that determine how atoms, ions, or molecules stack together in a solid material, much like how bricks fit together to build a wall. These arrangements repeat in all directions, forming the beautiful shapes we see in crystals. These repeating units are called Unit Cell. Let’s learn them in detail.
What is Unit Cell?
A unit cell is the smallest repeating structure in a crystal lattice. In other words, it’s like a building block that repeats over and over again to form the entire crystal structure. The arrangement of atoms or molecules within the unit cell determines the properties of the crystal.
Unit cells come in different shapes and sizes, depending on the crystal structure and system. They help us understand how atoms or molecules are arranged within a crystal and provide insights into the properties of the material.
Parameters of Unit Cell
Parameters of a unit cell describe its size and shape.
Lengths of Edges (a, b, c): These represent the lengths of the edges of the unit cell along the three crystallographic axes. Each edge may have a different length, defining the size of the unit cell in each direction.
Angles between Edges (α, β, γ): These parameters represent the angles between the edges of the unit cell. They define the orientation of the edges with respect to each other. α is the angle between edges b and c, β is the angle between edges a and c, and γ is the angle between edges a and b. These angles are usually measured in degrees.
Types of Unit Cell
There are two types of unit cells
- Primitive Unit Cell
- Non-Primitive Unit Cell
Primitive Unit Cell
Primitive Unit Cell are those in which atoms are at corners of the unit cell. One such example is simple cubic
Simple
- It has the simplest structure, with lattice points located at the corners of a cube.
- Atoms are only present at the corners.
Non-Primitive Unit Cell
In non-primitive unit cell, the atoms are present at corners as well as other parts of unit cell. There are three types of non-primitive unit cell
- Body Centered
- Face Centered
- Edge Centered
Body-Centered
- In addition to the lattice points at the corners there is one additional lattice point at the body center
- This structure is denser than the simple and is commonly found in some metals, such as iron and chromium.
Face-Centered
- In addition to the lattice points at the corners of the unit cell, there is one lattice point at the center of each face of the unit cell
- This structure is even denser than the body-centered and is found in many metallic elements, including aluminum, copper, and gold.
Edge Centered
- In addition to the lattice point at the corners of the unit cell, the lattice points are present on the center of each edge
End Centered
- In addition to lattice point at the corned of the unit cell, lattice points are present on the center of a pair of opposite face of the unit cell
Crystal Systems
The different parameters of unit cell generate different types of crystal structures called Crystal System. There are seven possible crystal with different configuration of unit cell parameters. The seven crystal systems are:
- Cubic
- Tetragonal
- Orthorhombic
- Monoclinic
- Triclinic
- Rhombohedral
- Hexagonal
Cubic Crystal System
In cubic crystals, all three crystallographic axes are of equal length and intersect each other at right angles (90 degrees). Examples include common substances like salt (sodium chloride) and diamonds.

Tetragonal Crystal System
In tetragonal crystals, two crystallographic axes are of equal length, while the third axis is different and perpendicular to the other two. This creates a rectangular prism shape. Rutile is an example of a mineral with a tetragonal crystal structure.

Orthorhombic Crystal System
Orthorhombic crystals have three axes of different lengths, all of which intersect at right angles. The resulting shape is a rectangular prism, but the sides are not necessarily equal in length. Sulfur and bismuth are examples of minerals with orthorhombic crystal structures.

Monoclinic Crystal System
Monoclinic crystals have three axes of different lengths, two of which intersect at oblique angles (not 90 degrees), while the third axis is perpendicular to the other two. This creates a prism shape with one angle that is not a right angle. Gypsum is an example of a mineral with a monoclinic crystal structure.

Triclinic
In triclinic crystals, all three crystallographic axes are of different lengths and intersect at oblique angles (not 90 degrees). This results in a crystal shape that lacks any form of symmetry. An example of a mineral with a triclinic crystal structure is microcline.

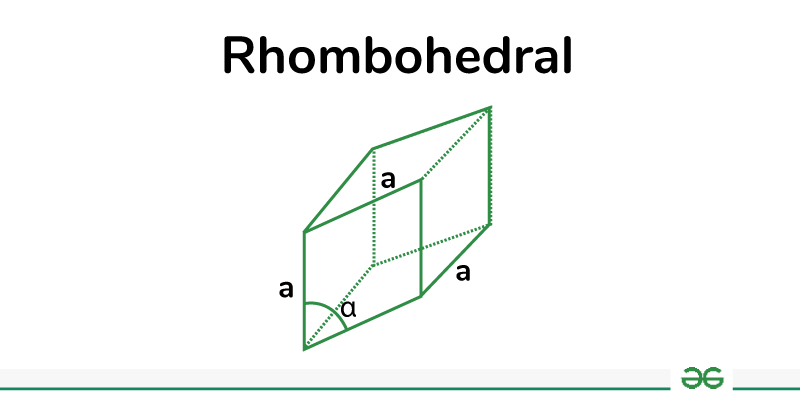
Rhombohedral
Rhombohedral crystals have three axes of equal length, all of which intersect at oblique angles (not 90 degrees). The resulting shape is a rhombohedron, which is like a cube that has been distorted into a shape with no right angles. Calcite is an example of a mineral with a rhombohedral crystal structure.
Hexagonal
Hexagonal crystals have four axes, three of which are of equal length and intersect at angles of 120 degrees, while the fourth axis is perpendicular to the other three. This creates a prism shape with a hexagonal base. Quartz and graphite are examples of minerals with hexagonal crystal structures.

These crystal systems help scientists classify and understand the structures of various minerals and materials based on their symmetry and atomic arrangement.
|
Crystal System
|
Axes Length
|
Angle Between Axes
|
Symmetry
|
Example
|
|
Cubic
|
All equal
|
All 90°
|
High
|
Salt (NaCl), Diamond
|
|
Tetragonal
|
Two equal, one different
|
All 90°
|
Medium
|
Rutile (TiO2)
|
|
Orthorhombic
|
All different
|
All 90°
|
Medium
|
Sulfur, Bismuth
|
|
Monoclinic
|
All different
|
Two 90°, One ≠ 90°
|
Medium
|
Gypsum
|
|
Triclinic
|
All different
|
All ≠ 90°
|
Low
|
Microcline
|
|
Hexagonal
|
Two equal, one different
|
All 90°, One 120
|
High
|
Quartz, Graphite
|
|
Rhombohedral
|
All equal
|
All ≠ 90°
|
High
|
Calcite
|
These seven crystal structure and their existence of various unit cell lead to fourteen possible lattice structure called Bravais Lattice. A lattice is arrangement of points in space. When atoms are placed on these lattice points it forms crystal. The crystal system chart is tabulated below:
.png)
Close Packing In Solids
Close packing in solids is like arranging a bunch of marbles as tightly as possible in a box. You try to stack them in layers so that they fill up the space efficiently. In close packing, the marbles are packed in such a way that they are as close together as possible, leaving very little empty space between them. This arrangement is important because it helps determine the properties of the material, such as its density and strength.
There are two common types of close packing: cubic close packing (CCP) and hexagonal close packing (HCP). Both types involve stacking layers of spheres in a way that minimizes empty space between them, resulting in a densely packed structure. Close packing is important in materials science because it influences the physical and mechanical properties of solid materials.
Types of Close Packing
There are two main types of close packing in solids:
Cubic Close Packing (CCP): It’s like arranging marbles in layers where the overall 3D arrangement appears to be a cubic structure. Each marble touches six other marbles. Then, the layers are stacked so that the marbles in the second layer fit into the spaces between the marbles in the first layer. This creates a structure with marbles at the corners and centers of each face of a cube.
Hexagonal Close Packing (HCP): It’s similar to CCP, but the arrangement of marbles in each layer forms a hexagon shape. The layers are stacked in a way that the marbles in the second layer fit into the spaces between the marbles in the first layer, following a repeating ABAB… stacking pattern. This creates a structure with a hexagonal shape.
Crystal Structure Conclusion
To sum up, crystal structures are like blueprints that define how atoms or molecules are arranged within crystals. These structures play a fundamental role in determining the physical, chemical, and mechanical properties of solids. These arrangements affect the properties of materials. By studying crystal structures, scientists can predict how substances will behave and even design new materials. Crystallography is essential for various fields and helps us understand nature better while also paving the way for new technologies.
Crystal Structures FAQs
What is a crystal structure?
A crystal structure refers to the specific arrangement of atoms, ions, or molecules within a crystalline material. It determines the overall shape and properties of the crystal.
How are crystal structures classified?
Crystal structures are classified into different types based on their symmetry and arrangement of atoms. The main types include cubic, tetragonal, orthorhombic, monoclinic, triclinic, hexagonal, and rhombohedral.
What are crystal axes?
Crystal axes are imaginary lines used to describe the orientation and symmetry of a crystal lattice. They help define the directionality of crystal growth and the arrangement of atoms within the crystal.
What is a unit cell?
A unit cell is the smallest repeating structure within a crystal lattice. It represents the basic building block of the crystal structure and helps describe the overall arrangement of atoms or molecules.
What are crystal planes?
Crystal planes are imaginary flat surfaces within a crystal lattice where the arrangement of atoms exhibits specific symmetrical properties. They are described using Miller indices and play a role in understanding crystal growth and material properties.
What is close packing in solids?
Close packing in solids refers to the arrangement of atoms, ions, or molecules in a way that maximizes their packing efficiency. The two main types of close packing are cubic close packing (CCP) and hexagonal close packing (HCP).
How do crystal structures impact material properties?
Crystal structures influence various material properties such as density, hardness, conductivity, and optical properties. Changes in the crystal structure can result insignificant alterations in material behavior.
What are some real-world applications of crystal structures?
Crystallography finds applications in various fields including materials science, pharmaceuticals, electronics, and geology. It is used to develop new materials, determine the structures of molecules, and understand geological formations.
Share your thoughts in the comments
Please Login to comment...