Amino Acids are essential for the human body. Amino acids are important in the formation of the structure of proteins. When a person eats foods that contain protein, the digestive system breaks down the protein into amino acids. The body then combines amino acids in various ways to perform physical functions. The body needs 20 amino acids to maintain good health and normal functioning. A healthy body can make only 11 amino acids on its own, but the other 9 amino acids cannot be synthesized by the body. The other 9 amino acids must be ingested from the other sources through food. A body that can make amino acids are called non-essential amino acids because it creates on its own. The amino acids which cannot make owned by the body are called essential amino acids because they are taken from other sources. Amino acids are an essential part of the functioning of various chemical and biological of our body. The different functions include transportation of molecules, building, and fixing of the tissues, development, and capability of enzymes, food digestion, and so on. These biomolecules are important elements for the development and advancement of individuals. There are around 300 amino acids that can be tracked down in nature. Be that as it may, just 20 amino acids are significant.
Amino Acids
Amino acids are organic compounds containing amine (- NH2), carboxyl (-COOH), and side chain—R group. The major elements of amino acids are carbon, hydrogen, nitrogen, and oxygen. Each molecule contains a central carbon atom, α-carbon to which both an amino and a carboxyl group are attached. The other two bonds of the α-carbon atom are bonded by a hydrogen (H) atom and the R group. All amino acids have the alpha carbon bonded to a hydrogen atom, carboxyl group, and amino group. The R group (side chain) varies among amino acids. The structure of amino acids is represented below.
Structure
Classification and Types
The different types of amino acids are classified based on
- Nutritional requirements
- R group
- Polarity and R group
- Distribution in protein
Based on nutritional requirements
Essential Amino Acids
Amino acids cannot be made by the body and should be taken from other sources in the form of food. Without this body cannot synthesize proteins. So, we need to take food that is rich in essential amino acids. The best sources of amino acids are found in animal proteins like beef, poultry, and eggs. They can be easily absorbed and used by our bodies. Foods that contain all nine essential amino acids are called complete proteins. The 9 essential amino acids are Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan, and Valine.
Essential Amino Acids with abbrev., Formula and Functions.
| Amino Acids |
Abbrev. |
Formula |
Functions |
| Valine |
Val |
C5H11NO2 |
It promotes muscle growth. |
| Leucine |
Leu |
C6H13NO2 |
Helps in the synthesis of proteins and growth hormones. |
| Histidine |
His |
C6H9O2N3 |
It is used to produce histamine and synthesis in red and white blood cells. |
| Isoleucine |
Ile |
C6H13NO2 |
It as a vital role in transporting oxygen to various parts of the body from lungs. |
| Lysine |
Lys |
C6H14N2O2 |
This is a representative essential amino acid, that forms antibodies, and hormones. Becomes insufficient when we are on diet. |
| Methionine |
Met |
C5H11NO2S |
Methionine helps to produce compounds that are crucial for the body. They are used for the treatment of kidney stones. |
| Phenylalanine |
Phe |
C9H11NO2 |
This essential amino acid helps to produce different useful amines. It boosts memory power, |
| Threonine |
Thr |
C4H9NO3 |
It forms active sites of enzymes. |
| Tryptophan |
Trp |
C11H12N2O2 |
Tryptophan is useful to produce amines, vitamin B3, and serotonin hormones. |
Non-essential amino acids
An amino acid that can be made by the own body itself. A healthy body can create on its own. Non-essential amino acids are mainly synthesized from glucose and other biological sources within your cells. Diet plays an important role if it does not provide enough of them, the body cannot synthesis essential amino acids. Because of that, you must include them in the foods you eat. The nonessential amino acids include Alanine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
Non-Essential Amino acids with abbrev., Formula and Functions.
| Amino Acids |
Abbrev. |
Formula |
Functions |
| Arginine |
Arg |
C6H14N4O2 |
It maintains the functions of blood vessels and other organs, maintaining a healthy immune system. |
| Alanine |
Ala |
C3H7NO2 |
It acts as an energy source for the liver. Production of glucose. |
| Asparagine |
Asn |
C4H8N2O3 |
It is close to the TCA cycle-place of energy generation, together with aspartic acid. Involves in development of nervous system. |
| Aspartate |
Asp |
C4H7NO4 |
It is present in asparagus in high amounts, a fast-acting energy source. Promotes the synthesis of other amino acids. |
| Cysteine |
Cys |
C3H7NO2S |
Cystine is an amino acid that can be deficient in infants. It acts as an antioxidant the acid antioxidant and gives resistance to the body. |
| Glutamic acid or Glutamate |
Glu |
C5H9NO4 |
Glutamate is a fast-acting energy source, involves in developing and functioning of the brain. |
| Glycine |
Gly |
C2H5NO2 |
It produces glutathione and porphyrin – a component of hemoglobin. Acts as a neurotransmitter. |
| Glutamine |
Gln |
C5H10N2O3 |
It maintains the functions of the gastrointestinal tract and muscles. Important in the synthesis of nucleic acids. |
| Proline |
Pro |
C5H9NO2 |
The main component of collagen. Regeneration of new skin. |
| Serine |
Ser |
C3H7NO3 |
Serine produces phospholipids and glyceric acid. Promotes muscle growth. |
| Tyrosine |
Tyr |
C9H11NO3 |
It produces amine and aromatic amino acids together with phenylalanine and tryptophan. Production of thyroid hormones. |
Based on R-Group
- Simple amino acids: These amino acids don’t have a functional group in their side chain. Example: valine, isoleucine.
- Hydroxy amino acids: Presence of hydroxyl group in their side chain. Example: serine, threonine.
- Sulfur-containing amino acids: They contain sulfur in their side chain. Example: cysteine, methionine.
- Aromatic amino acids: These amino acids contain a benzene ring in their side chain. Example: phenylalanine, tyrosine.
- Heterocyclic amino acids: Amino acids have a side chain ring that contains at least one atom other than carbon. Example: Tryptophan, histidine, proline.
- Amine group-containing amino acids: The derivatives of amino acids in which one of the carboxyl groups have been transformed into an amide group. Example: Asparagine, glutamine.
- Branched-chain amino acids (BCAAs): BCAAs are the amino acids that contain aliphatic side-chains with a branch. Example: isoleucine, valine.
- Acidic amino acids: Amino acids have a carboxyl group in their side chain. Example: Aspartic and Glutamic acid.
- Basic amino acids: Amino acids have an amino group in their side chain. Example: Lysine, Arginine.
- Amino acid: Amino acids consists of a secondary amine group. Example: Proline.
Polarity and R Group
- Amino acids with non-polar R group: These amino acids are hydrocarbons in nature
- Aliphatic R groups – Alanine, Valine, Leucine, Isoleucine, Proline.
- Aromatic groups—Phenylalanine, Tryptophan, Methionine (sulfur).
- Amino acids with polar but uncharged R Group: Amino acids are polar and have a neutral pH value. Example: Serine, Threonine, Tyrosine.
- Negatively charged amino acids: The side chain of amino acids has an extra carboxyl group with a dissociable proton. Example: Aspartic acid and Glutamic acid
- Positively charged amino acid: The side chain of an amino acid has an extra amino group, which gives basic nature to protein. Example: Lysine, Arginine, Histidine.
Distribution in protein
- Standard protein amino acids: The amino acids, which are recognized by ribozyme auto aminoacylation systems, are used to form proteins. Example: Methionine, Histidine, Lysine, Isoleucine, Leucine, Threonine, Tryptophan, Phenylalanine, and valine.
- Non-standard protein amino acids: Amino acids don’t form proteins, but have a vital role in metabolic intermediates. Example: Hydroxylysine, Carboxyglutamate.
- Non-standard nonprotein amino acids: These are the derivative of amino acids and have a role in metabolism. Example: Alpha amino butyrate, Ornithine, beta-alanine.
Physical Properties
- They are colorless.
- Amino acids are crystalline in nature.
- They are tasteless and sweet in nature.
- Amino acids have a melting point of above 200˚C.
- They are soluble in polar solvents like water, acetone, methanol, etc.
- They are insoluble in non-polar solvents like alkanes, acetic acids, chloroforms, etc.
- Amino acids have absorbance at 280 nm.
- The Molecular weight of amino acids is from 100 to 50,000Dt.
- They exhibit colloidal nature and denaturing properties.
Chemical Properties
Decarboxylation
Amino acids will undergo decarboxylation to form amines. Thus, amines are produced.
Examples:
- Histidine → Histamine + CO2
- Tyrosine →Tyramine + CO2
- Lysine →Cadaverine + CO2
Salt formation
The carboxyl group of amino acids can release hydrogen (H+) ions with the formation of Carboxylate (COO–) ions.
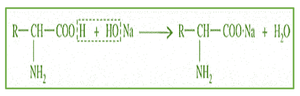
Reaction with alkanes
Esterification
When amino acids are reacted with an alcohol, they form an ester. The esters are volatile, in contrast to the form of amino acids.

Reaction with alcohol
Dimethyl Amino Naphtha Sulphonyl (DANSYl) Chloride
Amino acid reacts with DANSYl chloride reagent to form—Fluorescent DANSYl derivative.
Acylation
Amino acids react with acid chloride and acid anhydride in an alkaline medium to form phthaloyl amino acids.
Deficiency of Amino acids
Amino acids are the structure blocks of proteins, and proteins assume a key part in practically all life processes. In this way, it is important to remember every one of the nine essential amino acids for our day-to-day diet to keep a solid and legitimate capability of our body. The lack of amino acids might incorporate different neurotic problems, including Enema, Anemia, Insomnia, Diarrhea, Depression, Hypoglycemia, Loss of Appetite, Fat stored in the liver, Skin and hair-related issues, Headache, shortcoming, peevishness, and weariness.
Food Sources
Foods that incorporate high amounts of amino acids include
- Plant-based foods are broccoli, beans, beetroots, pumpkin, cabbage, nuts, dry fruits, chia seeds, oats, peas, carrots, cucumber, green leafy vegetables, onions, soybeans, whole grain, peanuts legumes, lentils, etc.
- Fruits that incorporate amino acids are apples, bananas, berries, figs, grapes, melons, oranges, papaya, pineapple, and pomegranates.
- Animal foods include dairy products, eggs, seafood, chicken, meat, pork, etc.
Frequently Asked Questions
Question 1: What is the importance of amino acids?
Answer:
The main importance of amino acids is:
- They are the structural part of the protein.
- They act as buffers in solutions by resisting changes in ph.
- They act as storage of nitrogen.
- Disulfide bonds are formed by the cysteine linking chains together.
- They help in electron transfer by the aromatic rings of Phenylalanine, Tyrosine, and Tryptophan.
- Amino acids lose amino groups to form glucose.
Question 2: What are non-protein amino acids?
Answer:
The α-amino acids are found in cells that are not integrated into proteins. Examples include ornithine and citrulline. Both of these mixtures are intermediates in the urea cycle. Ornithine is a metabolic forerunner of arginine and citrulline can be delivered by the breakdown of arginine. The last response produces nitric oxide, a significant molecule. Citrulline is the metabolic product. It is now and then utilized as a dietary enhancement to decrease muscle weariness.
Question 3: What are acidic and basic amino acids?
Answer:
Acidic amino acids are the amino acids that have a carboxyl group in their side chain or R-group. Examples of acidic amino acids are Aspartic and Glutamic acid. Basic amino acids are the amino acids which are having amino groups in their R-group or side chain. Examples of the basic amino acids are Lysine and Arginine.
Question 4: What are polypeptides?
Answer:
Amino acids are connected to one another by peptide bonds. The carboxyl group of one amino acid is joined to the amino group of the following, with the departure of a hydrogen molecule. Extra amino acids are included in the same way, by the development of peptide connections between the free carboxyl on the finish of the developing chain and the amino group of the following amino acid in the sequence. A chain comprised of only a couple of amino acids connected together is called an oligopeptide, while a normal protein, which is comprised of numerous amino acids is known as a polypeptide.
Question 5: What are the diseases caused due to deficiency of amino acids?
Answers:
The diseases caused due to deficiency of amino acids in the body are:
- Anemia.
- Insomnia or sleeplessness.
- looseness.
- Depression.
- Hypoglycemia.
- Loss of Appetite.
- Deposition of fat in the liver.
- Skin and hair problems.
- Fatigue, exhaustion.
Question 6: What is the isoelectric point of amino acids?
Answer:
In amino acids with uncharged side-chains, the zwitterion prevails at pH values between the two pKa values. However, coincides in balance with limited quantities of net negative and net positive particles. At the midpoint between the two pKa values, the following measure of net negative and hint of net positive particles balance, so that the typical net charge of all structures present is zero. This pH is known as the isoelectric point.
Question 7: What are Standard and Non-Standard amino acids?
Answer:
The 20 amino acids that are encoded directly by the standard genetic code are called standard or canonical amino acids. They are proteinogenic which means they are the structural parts of proteins or participate in the synthesis of protein. The other amino acids are called nonstandard or non-canonical. Most of the nonstandard amino acids are also non-proteinogenic because they cannot create protein, but two of them are proteinogenic.
Share your thoughts in the comments
Please Login to comment...