Why does Carbon Always Form Covalent Bonds?
Last Updated :
12 Oct, 2021
As carbon plays such an important role in living organisms’ chemistry, all are carbon-based. This suggests that carbon atoms, bound to other carbon atoms or other elements, are the basic building blocks of many, if not all, of the compounds, found only in living beings. Other atoms play vital roles in biological molecules, but carbon is unquestionably the “foundation” element for living organisms. Carbon atoms’ bonding characteristics are responsible for their significant importance.
Carbon
Carbon with atomic number 6, has the configuration (2,4) with four valence electrons in the valence shell. To attain stability, carbon needs to have the noble gas configuration of inert or noble gases which are nearest to it.
The carbon atom is one of the few elements that have the ability to establish large networks of covalent connections with both other elements and itself. Carbon is neither an electropositive nor an electronegative element due to its position in the second horizontal row of the periodic table; as a result, it is more likely to share electrons than to gain or lose them.
Because it can mix with itself and many other elements, carbon is a relatively common “ingredient” of matter. It may produce a wide range of compounds, with sizes ranging from a few atoms to thousands of atoms. Carbon is the only element that can produce so many distinct compounds, and there are millions of them known.
Valence Electrons in Carbon
Carbon is a nonmetal in the periodic table’s group 14. Carbon, like the other elements in Group 14, has four valence electrons. The electrons in an atom’s outer energy level that are engaged in chemical bonds are known as valence electrons. The electron dot diagram in the Figure below depicts the valence electrons of carbon.

Carbon with four valence electrons
To fill its outer energy level, carbon requires four more valence electrons, for a total of eight valence electrons. The most stable arrangement of electrons is a whole outer energy level. Four covalent bonds can be formed by Carbon. Chemical bonds between nonmetals are known as covalent bonds. Two atoms share a pair of electrons in a covalent bond. Carbon shares four pairs of electrons by establishing four covalent bonds, thus filling its outer energy level and ensuring stability.
How Carbon Forms Covalent Bonds?
Gas is said to be a noble gas in nature if it has 8 electrons in its valence shell or two electrons in its valence shell in the case of Helium. Noble gases are also called inert gases or stable gases because they are stable in nature. Every atom tries to attain a noble gas configuration through bonding.
Noble Gases nearest to Carbon are Helium (He = 2) and Neon (Ne = 2, 8) are the inert gases nearest to carbon. Now, carbon has two choices to attain this configuration either by gaining four electrons (attaining Ne configuration) or by losing 4 electrons (attaining He configuration). But both of the choices with the Ionic Bond Formation are not feasible because:
- Formation of Carbon Anion by the gain of four electrons: By gaining four electrons, carbon becomes a C-4 Anion.

Carbon Anion
Anions are negatively charged ions formed when the atom has more electrons than protons. Here, Carbon has 6 protons and 10 electrons.
Although carbon attains Ne configuration it becomes difficult for the nucleus to hold ten electrons with six protons. So, it’s unstable and this bonding doesn’t take place.
- Formation of Carbon Cation by the loss of four electrons: By losing four electrons, carbon becomes C+4 Cation.

Carbon Cation
Cations are positively charged ions formed when the atom has more protons than electrons. Here, Carbon has 6 protons and 2 electrons
Although carbon attains He configuration it becomes difficult for the nucleus to hold two electrons with six protons. So, it’s unstable and this bonding doesn’t take place.
These two reasons don’t support the formation of Ionic Bonds in the case of Carbon. So, to its rescue Carbon completes its octet by sharing electrons and completing its octet. Such type of bonding where element completes their octet by sharing electrons is known to be Covalent in nature.
Thus, Carbon always forms Covalent Bonds.
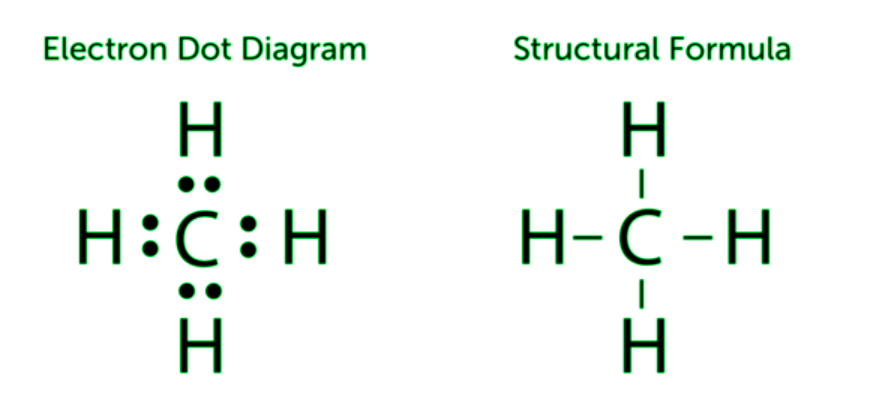
Covalent bonds can be formed between carbon atoms or between carbon atoms and the atoms of other elements. Carbon and hydrogen frequently form bonds. Hydrocarbons are compounds that simply contain carbon and hydrogen. A hydrocarbon is something like methane (CH4), which is modelled in the diagram below. One carbon atom forms covalent connections with four hydrogen atoms in methane. All of the shared valence electrons are depicted on the left in the diagram below. Each pair of shared electrons is represented by a dash (–) in the figure on the right in the Figure below, which is called a structural formula.
Sample Questions
Question 1: What are the atomic number and atomic configuration of Carbon?
Answer:
Carbon possesses atomic number 6 and has configuration 2,4.
Question 2: Can carbon form Ionic Bonds? If yes, give example.
Answer:
Carbon cannot form Ionic Bonds. It only forms Covalent bonds with other atoms.
Question 3: Define Covalent Bonding.
Answer:
Bonding where there is no loss or gain of electrons and bond formation takes place by sharing electrons is called bonding having covalent nature or simply covalent bonding.
Question 4: Give examples of Carbon with Covalent bonds formation taking place.
Answer:
CO2, CCL4, CH4 are all examples of Carbon making Covalent Bonds with other atoms.
Question 5: Explain the bond formation of CO2. What type of bonding takes place between the atoms of CO2? Diagrammatically explain its bond formation.
Answer:
The nature of bonding between the atoms of CO2 is covalent in nature. Carbon and oxygen share electrons among them and there is no give or take of electrons. Carbon has 4 electrons in its valence shell shares its electrons with Oxygen having 6 electrons in its valence shell. In this way, there is sharing of electrons between atoms which takes place in CO2.
The diagrammatic representation of CO2 Bond formation is represented below:

Formation of CO2 by Covalent Bonding of Carbon with Oxygen
Share your thoughts in the comments
Please Login to comment...