What is Freezing?
Last Updated :
14 Jul, 2021
Change of state in a substance is a physical change in a matter. These changes accommodate reversible changes, that is the changes that can be changed from one physical state to another. It does not involve any chemical changes. The changes occur due to variations observed in the temperature or pressure of a substance. For instance, on increasing the temperature the intra-molecular interaction among the particles rise, therefore, the substance move more freely. On decreasing the temperature, the substances settle down in a fixed and rigid structure. Governed by the three states of matter, the different processes encountered in the cycle are melting, freezing, sublimation, deposition, condensation, and vaporization.

The changes in phases of a substance result in the change in the composition of a substance. Solids have rigid structures and fixed volumes. This is because in solids the intramolecular forces of attraction are stronger than that of the hydrogen bonds. This is in complete comparison to the state of gas which neither has definite volume nor a definite shape. Because the particles are loosely bonded to each other and allowed to move and transmit in all directions. It expands until it occupies the complete container in which it is placed.
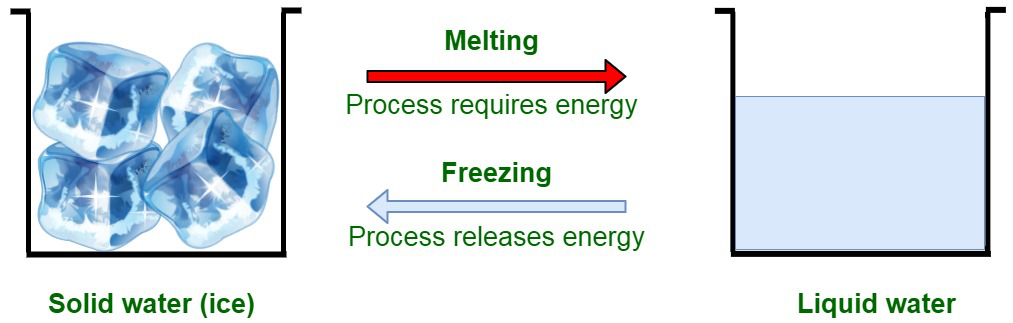
When the heat is released through the system the transition occurs from the gaseous state towards the solid-state. For instance, ice upon cooling results in the transformation to the solid (ice) state.
Freezing
The particles in the liquid state of a solvent are loosely coupled with respect to each other. This is an indicator of the weak forces of attraction among the particles than of the solid-state. Freezing is a state of liquid where the temperature of the system is lowered below the freezing point of the solid. Therefore, it is a phase transition where a liquid turns into a solid-state. To summarize, freezing is the solidification phase change of liquid contents of a substance, which primarily occurs due to the cooling of a substance.
Freezing is an exothermic reaction, that is the heat is released during the freezing process. Freezing is also known as solidification.
Examples of freezing:
- Freezer
- Anti-freezer
- Frozen food
- Snowfall
- Snowflakes
- Sea ice
Why freezing occurs?
Freezing of a solid occurs when the heat energy transferred to the molecules of the system during the process of conversion from solid to the liquid state is comparatively greater than its potential energy holding the particles together in the form of a crystal lattice of a solid. This potential energy of the substance indicates the lattice energy of the solid.
When we decrease the temperature of a substance, the heat is released from the system. This results in the extraction of heat and lowering of pressure. The particles forming the substance lose their kinetic energy and approach each other more closely. This is further followed by the increase in the potential energy of the system. The system slowly attains a stable state. The conversion to solid therefore occurs.
Freezing point
Freezing point of a substance is the temperature at which a liquid turns to a solid-state. All the liquids possess a characteristic at which they freeze and transform to the solid-state. The increase in pressure increases the freezing points. Mixtures and organic compounds possess a lower freezing point in comparison to the melting point.
Different liquids have different freezing points as illustrated in the table below:
| Solvent |
Formula |
Freezing Point (°C) |
| Water |
H2O |
0.0 |
| Acetic acid |
CH3COOH |
17.0 |
| Benzene |
C6H6 |
5.5 |
| Chloroform |
CHCl3 |
-63.5 |
| Ethanol |
C2H5OH |
-114.7 |
| Phenol |
C6H5OH |
43.0 |
Factors affecting the freezing point of a Liquid
The various factors affecting the freezing point of a liquid are as follows :
- Types of molecules
The particles forming a liquid affect the freezing point of the liquid. The intermolecular forces of attraction are directly proportional to the freezing point. For instance, the intermolecular forces of attraction between molecules of the liquid are weak, therefore, its freezing point is low.
- Pressure
When the pressure of a substance is lowered below 1 atm, freezing occurs. However, in the case of water, the higher the pressure, the lower is the freezing point, that is, they are inversely proportional to each other.
- Types of changes
Physical and chemical changes affect the freezing point of a substance. For instance, the freezing point of a solvent decreases upon mixing another soluble substance in it. This is a chemical change. Similarly, altering the pressure lowers the freezing point.
Heat of Fusion
The heat is extracted from the liquid contained in the system in order to freeze it. Some of the liquids can be supercooled—that is, these liquids can be cooled down below the freezing point. This is the state where solid crystals do not form. When we place a seed crystal into a supercooled liquid, the freezing is initiated and triggered. When the heat of fusion is released, the temperature rises rapidly to the freezing point.
Supercooled Liquid
Supercooling is the process of cooling the liquid, beyond its freezing point such that the liquid doesn’t change to the solid-state. It is the phase at which the liquid does not solidify even on subjecting it to a point beyond its freezing point. For most of the substances, the freezing and melting points of a substance are equivalent. Certain substances get cooled beyond their freezing point while retaining their liquid phase. These are termed supercooled liquids.
Examples- The clouds at high altitudes are a collection of supercooled water droplets, all collaborated below their freezing point.
Sample Questions
Question 1: Differentiate between the freezing point and melting point?
Answer:
| |
Freezing Point |
Melting Point |
| Definition |
The temperature at which a liquid changes its state to a solid. |
The temperature at which solid changes into the liquid state. |
| Purity of end product |
Similar to the melting point of the same substance, however, may slightly vary |
Similar to the freezing point of the same substance however may slightly vary |
| Entropy |
Decreases |
Increases |
Question 2: Which elements have the highest freezing point?
Answer:
The highest freezing point is of the element Tungsten with the highest fusion rate, corresponding to 3695 K.
Question 3: A glass container containing ice has water droplets collected on the outer surface. Explain.
Answer:
Cold water contained in a glass container cools down. The water vapour contained in the air comes in contact with wall of the glass container. Owing to the low temperature the vapour, the liquid forms in the form of water droplets on the outer surface. This process is known as condensation.
Question 4: What is freezing point depression?
Answer:
Freezing-point depression is termed as the decrease in the temperature at which a substance freezes, which occurs when a smaller amount of another, non-volatile substance is added to the system. This concept can be visualized easily in daily life include adding salt into water (used in ice cream makers and for de-icing roads), mixing of two impurities into a finely powdered drug or alcohol in water.
Question 5: Explain why water as ice has a cooling effect, whereas water as steam may cause severe burns.
Answer:
In the solid-state of ice, the water molecules possess low energy whereas in the case of steam the water molecules possess high energy. The high energy of water vapor in the form of steam gets transferred to the body and may cause burns, whereas, in the case of ice, the reaction is exothermic, where the water molecules take energy from the body, and thus, a cooling effect is felt.
Question 6: Formulate for freezing point depression and its examples.
Answer:
Freezing point depression = ΔTF = KF × b × i
where,
ΔTF = freezing-point depression
KF = cryoscopic constant
b = molality
i = van ‘t Hoff factor.
Examples:
- Salting Icy Roads.
- Natural Antifreeze: Survival in Cold Weather.
- Radiator fluids in Automobiles.
Question 7: Differentiate between the freezing point and boiling point?
Answer:
Boiling Point:
- The temperature at which the vapour pressure of a liquid is considered equivalent to the pressure surrounding the liquid where it is converted to vapour.
- Different liquids possess varying boiling points based on their ‘environmental pressure’.
- For example, the B.P of ‘water’ is 100o C
Freezing Point:
- The temperature at which a substance undergoes the transformation from the liquid into solid.
- The freezing point and pressure are directly proportional to each other.
- For example, the freezing point of water is 0o C
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...