Mixtures
Last Updated :
01 Apr, 2024
A mixture is a compound that is made up of two more chemical compounds or substances that do not combine together chemically. It is the physical combination of two or more substances that can retain their individual identities while they are mixed to form solutions, suspensions, or colloids.
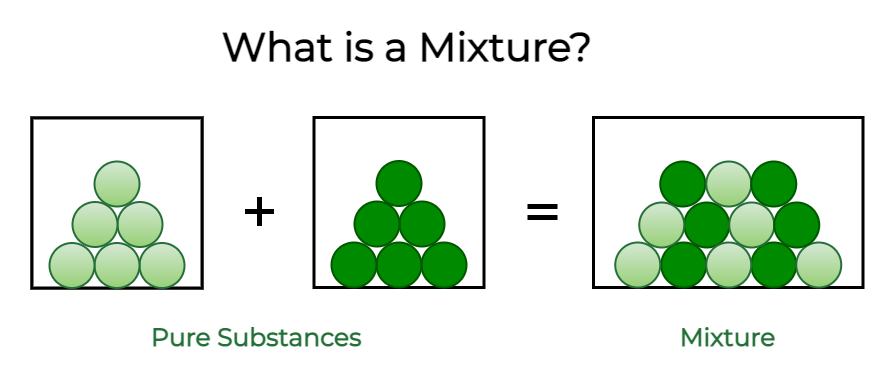
In this article, we will learn about Mixtures in detail, including its Properties, Examples, and Types in detail.
What is a Mixture?
Mixtures are substances that are made up of two or more different types of substances. Physical means can be used to separate them. A solution of salt and water, a combination of sugar and water, various gases, air, and so on are examples.
The different components of any combination do not unite through any chemical changes. As a result, the components retain their distinct characteristics.
Properties of Mixtures
The various properties of Mixtures are,
- The components of a mixture are not chemically bound together.
- Mixture can be both Homogenous and Heterogenous.
- The components of a mixture are not mixed in proper proportions.
- All the components of the mixture retain their original identities.
- Boling pint and melting point of a mixture is not constant.
- No chemical change occurs when a mixture is formed.
- Mixture can be separated using Physical Methods.
- Mixtures can exist in any state of matter, i.e. Solid, Liquid or Gas.
Examples of Mixtures
We see various mixtures in our daily life and some of the examples of mixtures are,
- Smog is a mixture of Smoke and Fog.
- Cement is a mixture of Sand, Water and Gravel.
- Sea Water is a mixture of Water and Salt.
- Soil is a mixture of Minerals, Air, Organic materials, Water, and Living Organisms.
- Blood is a mixture of Plasma, White Blood Cells, Red Blood Cells, and Platelets.
- Gasoline is a mixture of Hydrocarbons, Petroleum, and Fuel Additives.
- Brass is an Alloy and is a mixture of metals like Zinc and Copper.
- Nichrome is also an Alloy and is a mixture of metals like Chromium, Iron, and Nickel.
- Bleach is a mixture of Caustic Soda, Chlorine, and Water.
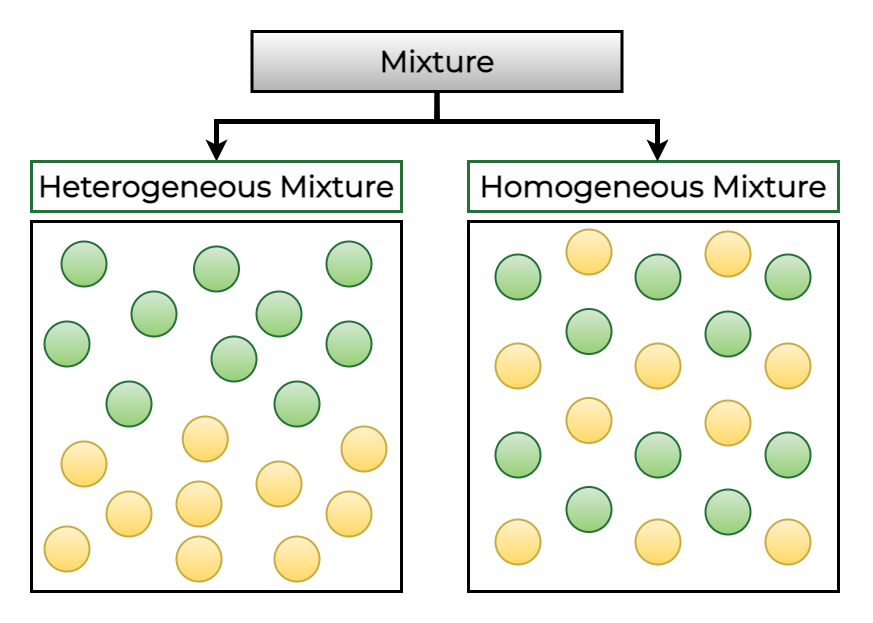
Types of Mixtures
Mixtures are broadly classified into two main categories. That are
- Homogeneous Mixtures
- Heterogeneous Mixtures
Now let’s learn about both these mixtures in detail.
Homogeneous Mixture
Homo means same. The mixtures in which the components have a uniform distribution throughout the mixture are known as homogeneous mixtures.
For example, salt and water is homogeneous mixture as the taste of the water will be the same if you take a sip from any portion of water. This shows that salt is uniformly distributed in the mixture.
e.g. Salt and Water, Sugar and Water, Alcohol and Water, etc.
Properties of Homogeneous Mixtures
Various properties of the Homogenous Mixture are,
- These have a uniform distribution of components throughout the mixture.
- The centrifugal force cannot be used to separate the components.
- Homogeneous mixtures do not exhibit the Tyndall Effect i.e. the scattering of light by the particles in the medium when a light beam is an incident on the mixture. The path of light becomes visible due to the scattering of the light beam.
- The particle size is <1nm.
- All the solutions are homogeneous mixtures.
Homogeneous Mixture Examples
Some of the examples of the Homogenous Mixture are,
- Air
- Soft Drink
- Cooking Gas
- Coffee
- Cement
- Vinegar, etc.
Heterogeneous Mixture
Hetero means different. The mixtures in which the components do not have a uniform distribution throughout the mixture which means in which the components are unevenly distributed are said to be heterogeneous mixtures.
For example, sand and water is an example of the heterogeneous mixture as sand does not distribute uniformly in water. e.g. Sand and water, Sugar and salt, Ice in water, etc.
Properties of Heterogeneous Mixtures
Various properties of the Heterogenous Mixture are,
- The components of heterogeneous mixture do not uniform distribute throughout the mixture
- You can draw a boundary between the components by just looking at the mixture.
- The particle size ranges between 1nm to 1 μm.
- They can exhibit the Tyndall effect.
Heterogeneous Mixture Examples
Some of the examples of the Heterogeneous Mixture are,
- Iron Ore
- Granite
- Milk and Cereal
- Sugar and Salt
- Water and Oil
- Rice and Beans
- Water and Sand, etc.
Also Read
Difference Between Mixture and Compound
The difference between compounds and mixtures are described below,
|
Mixture vs. Compound
|
|
Mixture
|
Compound
|
|
Two or more substance physically combined Together
|
Two or More substance chemically combined together
|
|
Substances can combine in any ratio
|
Elements always combine in a fixed ratio
|
|
Substances can can be separated by physical process
|
Elements separated from compounds by chemical decomposition
|
|
Property of Mixture resembles to the similar property of each constiuent substance
|
Property of Compound is different from constituent elements
|
|
It is Impure Substance
|
It is Pure Substance
|
|
Example – Brine Solution, Sugar-Water Solution
|
Example -Sodium Chloride, Calcium Carbonate
|
Read More
Mixtures – FAQs
What is a Mixture in Chemistry?
Mixture in Chemistry is defined as a compound that is made up of two more chemical components that are not chemically linked with each other.
What is Homogeneous Mixture?
Homogeneous mixtures are those mixtures in which the substances are uniformly distributed throughout the mixture. Example: Sugar and water, salt and water, etc.
What is Heterogeneous Mixture?
Heterogeneous mixtures are those mixtures in which there is no uniform distribution of substances. All the substances are unevenly distributed in the mixture. Concentration of a substance may be high or low at certain points in the mixture. Example: Sand and water, Salt and sand, etc.
What is the Difference between Homogeneous and Heterogeneous Mixtures?
The following are the differences between homogeneous and heterogeneous mixtures:
| Homogeneous |
Heterogeneous |
| Substances are uniformly distributed throughout the mixture. |
Substances are unevenly distributed throughout the mixture. |
| They do not exhibit Tyndall effect. |
They exhibit Tyndall effect only in case of colloids. |
What is Tyndall Effect?
Tyndall Effect refers to the scattering of light by the particles of a medium when a beam of light is incident on it. As a result of scattering the path of light becomes visible.
What is Size of Particles for Solutions, Colloids, and Suspensions?
The particle size for solutions, colloids and suspensions is as follows:
- Solutions: <1 nm
- Colloids: 1 nm to 1 μm
- Suspensions: >1 μm
What is Brownian Motion?
Brownian Motion is the random movement of the particles suspended in a suspension. It is the continuous and random zig-zag movement of the particles in the medium.
What is a Pure Substance?
Pure substance is the one which is composed of particles of only one element or compound. Example: Sugar, Salt, etc.
What are Components of Following Mixtures
- Aqua Regia
- Brass
- Water Gas
- Bronze
- Baking Soda
- Aqua Regia is a mixture of concentrated hydrochloric acid and concentrated nitric acid.
- Brass is a mixture of mixture of metals like Zinc and Copper.
- Water Gas is a mixture of Carbon Monoxide and Hydrogen.
- Bronze is a mixture of Copper and Tin.
- Baking Soda is a mixture of Carbon, Sodium, Hydrogen and Oxygen molecules.
Is Mixture a Homogenous?
No, Mixture is not necessarily Homogenous in nature. It can be both Homogenous or Heterogenous.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...