Types of Chemical Bonds as the name suggest explore the different types of chemical bonds which exist and form in the domain of chemistry. Bons such as Ionic, Covalent, Hydrogen, and Metallic include polar and non-polar bonds. Understanding these bonds is necessary as without knowing how these bonds are formed it is very difficult to analyse anything in the chemistry. In this article, we will explore all the different types of chemical bonds, their formation, properties, and examples as well. So, let’s begin our journey of learning about chemical bonds and their various types.
What is Chemical Bond?
Chemical bonding is the process of uniting two or more atoms by the redistribution of electrons, resulting in each atom achieving a stable electronic state.
To achieve security, each of the atoms completes its duplet or octet by obtaining the nearest tolerable gas electronic arrangement. It’s usually performed by the formation of composite connections between particles.
Types of Chemical Bonds
Chemical bonds are classified into four groups:
Ionic Bond
The octet rule states that an atom is most stable when its valence shell has eight electrons. Atoms in a solid state lose, acquire, or share electrons in their valence shell to achieve stability. A cation is formed when an atom loses one or more valence electrons to become a positively charged ion, whereas an anion is formed when an atom receives electrons and becomes a negatively charged ion.
An electrical force of attraction known as the Ionic Bond emerges when electrons are completely transferred from the positively charged cation to the negatively charged anion.
The octet rule is satisfied when valence electrons are exchanged, allowing ions to reach electronic configurations of the nearest noble gases. A positive superscript charge (+) to the right of the atom denotes a cation. A negative superscript charge (–) to the right of the atom represents an anion.
Note: Learn more about Valence Bond Theory by visiting the attached link.
Example of Ionic Bond
- When a sodium atom loses one electron, it gains one more proton than it loses an electron, giving it a positive (+1) charge overall. The chemical symbol for sodium-ion is Na+1 or simply Na+.
- When a chlorine atom gets an additional electron, it forms the chloride ion Cl–. Because of the octet rule, these ionic species are more stable than the atom.
Formation of an Ionic Bond
An ionic or electrovalent bond is a chemical bond produced between two atoms by the transfer of one or more electrons from an electropositive or metallic element to an electronegative or non-metallic element. The electrical arrangement of the sodium atom is 2,8,1, as we know. In its outermost shell, it just possesses one electron. It gets the inert gas electrical structure of Neon by donating this electron (2,8).
The electrical arrangement of the chlorine atom, on the other hand, is 2,8,7. It simply needs one more electron to complete its octet and achieve the inert configuration of Argon (2,8,8).
To remove an electron from its outermost shell and produce the positively charged sodium ion Na+, the sodium atom requires an amount of energy equal to its ionization energy of roughly 496kJ/mol. This is referred to as an endergonic process since it consumes energy. The chlorine atom, on the other hand, which is missing one electron, takes an electron and releases energy equivalent to its electron affinity, i.e. To produce a chloride ion, 349kJ/mol is required. The Exergonic process gets its name from the fact that energy is released during the process.
As a result, the oppositely charged ions create electrostatic forces of attraction, which are powerful forces of attraction. These forces drive the ion closer together, resulting in the formation of an ionic bond. Electrostatic forces are hence the foundation of an ionic connection.
What is Electrovalency?
The amount of atoms lost or acquired by one atom is referred to as electrovalency. In NaCl, the electrovalency of sodium and chlorine is one. As a result, they are monovalent. Calcium provides two valence electrons to generate calcium ions (2,8,8), and oxygen obtains two electrons to form oxide ions in the creation of Calcium oxide (CaO) (2,8). Calcium and oxygen have the same electrovalency, i.e. they are divalent. The oppositely charged ions are formed once the electrons are transferred. The development of an ionic link between sodium and fluorine atoms is seen here.

Conditions for the Formation of Ionic Bond
The formation of an ionic bond occurs under specific conditions that involve the interaction between atoms with significantly different electronegativities. Here are the key conditions for the formation of an ionic bond:
- Electronegativity Difference: Ionic bonding typically occurs between elements with a large difference in electronegativity. Electronegativity is a measure of an atom’s ability to attract electrons toward itself in a chemical bond. When the electronegativity difference between two atoms is high (usually greater than 1.7 on the Pauling scale), the bond tends to be ionic.
- Transfer of Electrons: In an ionic bond, one atom (usually a metal) tends to lose electrons and form a positively charged ion (cation), while the other atom (usually a nonmetal) tends to gain those electrons and form a negatively charged ion (anion). The transfer of electrons occurs to achieve a more stable electron configuration.
- Stable Octet: The primary driving force for the formation of ionic bonds is the attainment of a stable electron configuration, often resembling that of a noble gas. Atoms gain or lose electrons to achieve a complete outer shell with eight valence electrons (except for hydrogen and helium, which can achieve stability with two valence electrons). By transferring electrons, atoms acquire the electron configuration of the nearest noble gas, which enhances their stability.
- Coulombic Attraction: After electron transfer, the resulting oppositely charged ions are held together by strong electrostatic forces of attraction. The positive charges of cations are attracted to the negative charges of anions, creating a strong bond.
- Crystalline Structure: Ionic compounds typically adopt a crystal lattice structure, with ions arranged in a repeating pattern. The attractive forces between ions extend throughout the entire crystal lattice, resulting in a three-dimensional network held together by ionic bonds.
Writing Formula of an Ionic Compound
The following two requirements must be met in order to derive the chemical formulae of ionic compounds:
- For optimum stability, the cation and anion should obey the octet rule.
- Ions should unite in such a way that their charges balance out and the ionic composition as a whole is neutral.
The number of electrons provided or received is represented by the charges on the anion and cation. In ionic bonding, the compound’s net charge must be zero.
Properties of an Ionic Bond
There are various properties of the Ionic Bond, some of which are as follows:
- Ionic compounds are formed when a metal donates one or more electrons to a nonmetal.
- The metal loses electrons to form a positively charged ion, called a cation. The nonmetal gains electrons to form a negatively charged ion, called an anion.
- At room temperature, most ionic compounds are crystalline solids. The ions are arranged in a three-dimensional structure, with the cations and anions alternating.
- Ionic compounds have high melting points because the ions are held together by strong electrostatic forces. The melting point of an ionic compound is the temperature at which the forces of attraction between the ions are overcome and the ions are able to move freely.
- Ionic compounds are typically non-conductors of electricity in the solid state. However, when they are melted or dissolved in water, the ions are able to move freely and the compound becomes a conductor of electricity.
- Ionic compounds are soluble in polar solvents, such as water. This is because the polar water molecules can interact with the ions in the compound. The negative end of the water molecule interacts with the cations, while the positive end of the water molecule interacts with the anions. This interaction helps to break down the ionic compound and dissolve it in water.
- Ionic compounds are brittle. This is because the strong electrostatic forces between the ions hold the crystal together in a rigid structure. If the crystal is subjected to a force, the ions can be pulled apart and the crystal will break.
These properties can be summarized in the following table:
|
Property
|
Description
|
|
Melting point
|
High
|
|
Boiling point
|
High
|
|
Solubility
|
Soluble in polar solvents
|
|
Conductivity
|
Non-conductive in the solid state and
Conductive in the molten or dissolved state
|
|
Brittleness
|
Brittle
|
Covalent Bond
A covalent bond is a type of chemical bond formed between two atoms by the sharing of electrons. In simple terms, a covalent bond is the exchanging of electrons between particles to achieve the honorable gas configuration of individual iotas. In a covalent bond, atoms share one or more pairs of electrons in order to achieve a more stable electron configuration.
Characteristics of Covalent Bond
Key characteristics of covalent bonds are as follows:
- Electron Sharing: Covalent bonds occur between nonmetal atoms or between nonmetal and hydrogen atoms. In this type of bond, each atom contributes one or more electrons to a shared electron pair. The shared electrons are attracted to the nuclei of both atoms, holding them together.
- Electronegativity: In a covalent bond, the electronegativity difference between the two atoms is relatively small (usually less than 1.7 on the Pauling scale). This means that neither atom completely loses or gains electrons, but instead, they share the electrons in a more equal manner.
- Stable Electron Configuration: By sharing electrons, both atoms can achieve a stable electron configuration. They strive to attain a complete outer electron shell with eight valence electrons (except for hydrogen and helium), known as the octet rule. Sharing electrons allows the atoms to fill their valence shells without undergoing a complete electron transfer.
- Molecular Orbitals: In covalent bonding, the shared electrons occupy molecular orbitals that extend over both atoms. These molecular orbitals are formed by the overlapping of atomic orbitals from the participating atoms. The formation of molecular orbitals allows for the delocalization of electrons, resulting in the stability of the molecule.
- Multiple Bonds: In covalent bonding, there can be the formation of single, double, or triple bonds based on the sharing of pair of electrons.
Types of Covalent Bonds
The covalent bond can be categorized into the following categories based on the number of shared electron pairs:
- Single Covalent Bond
- Double Covalent Bond
- Triple Covalent Bond
Let’s understand these types as follows:
Single Bonds
When just one pair of electrons is shared between the two participating atoms, a single bond is established. One dash is used to symbolize it (-). Although it has a lower density and is weaker than double and triple bonds, this type of covalent bond is the most stable.
For Example, One Hydrogen atom has one valence electron and one Chlorine atom has seven valence electrons in the HCL molecule. By sharing one electron, a single bond is established between hydrogen and chlorine in this situation.

Double Bonds
When two pairs of electrons are shared between the two participating atoms, a double bond is established. Two dashes (=) are used to indicate it. Double covalent bonds are substantially more powerful than single covalent bonds, but they are also less stable.
Example: One carbon atom has six valence electrons and two oxygen atoms have four valence electrons in a carbon dioxide molecule.
Carbon shares two of its valence electrons with one oxygen atom and two with another oxygen atom to complete its octet. CO2 has two double bonds because each oxygen atom shares its two electrons with carbon.

Triple Bond
When the two participating atoms share three pairs of electrons, a triple bond is established. The least stable forms of covalent bonds are triple covalent bonds, which are indicated by three dashes (≡).
For Example: Each nitrogen atom with five valence electrons contributes three electrons to form three electron pairs for partaking in the construction of a nitrogen molecule. The two nitrogen atoms create a triple bond as a result.

Based on Polarity, covalent bonds can be classified as follows:
- Polar Covalent Bond
- Nonpolar Covalent Bond
Let’s understand these types of covalent bonds as follows:
Polar Covalent Bond
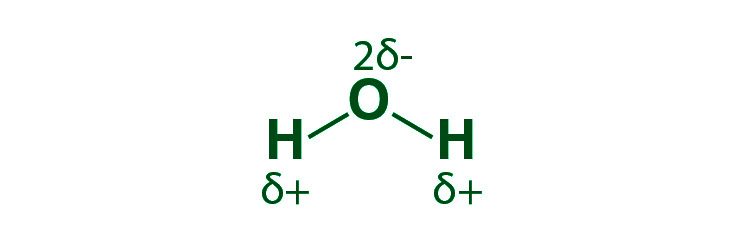
Because the electronegativity of the combining atoms differs, this sort of covalent bond develops when electrons are shared unequally. The attraction for electrons will be larger for more electronegative atoms. Between the atoms, the electronegative difference is more than zero but less than 2.0. As a result, that atom’s shared pair of electrons will be closer.
Example, An uneven electric potential causes molecules to form hydrogen bonds. The hydrogen atom interacts with the electronegative fluorine, hydrogen, or oxygen in this situation.
Nonpolar Covalent Bond
- When atoms share an equal number of electrons, this sort of covalent connection is produced. The difference in electronegativity between two atoms is zero. It happens when the atoms joining have a comparable electron affinity (diatomic elements).
Example, Gas molecules such as hydrogen and nitrogen include nonpolar covalent bonds.
Polarization of Covalent Bonds
The electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond in sigma bonds between two distinct atoms. As a result, the bond develops a permanent dipole, and the covalent bond is said to be polarised.
Properties of Covalent Bond
There are various properties of covalent bonds, some of which are as follows:
- Because of the modest intermolecular forces of attraction, covalent compounds have low boiling and melting points. At ambient temperature, these chemicals exist in all three physical states. While covalent interactions between atoms are fairly strong, intermolecular forces or attractions between molecules/compounds are comparatively moderate. When a smaller quantity of energy is applied to covalent bonds, molecules can split from one another. As a result, these chemicals are extremely volatile.
- The enthalpy of fusion is the amount of energy required to melt one mole of a solid material under constant pressure. The enthalpy of vaporization is the amount of energy necessary to vaporize one mole of a liquid at constant pressure. It takes just 1 to 10 times the amount of heat to alter the phase of a molecular covalent compound as it does to change the phase of an ionic molecule.
- Weak intermolecular forces of attraction characterize covalent compounds, causing them to adopt the forms of gases, liquids, and soft solids. There are exceptions, as with many features, particularly when molecular compounds take on crystalline forms.
- The majority of combustible compounds are made up of hydrogen and carbon atoms. In the presence of oxygen, these chemicals may easily undergo combustion processes, producing carbon dioxide and water. Because carbon and hydrogen have similar electronegativities, they are found in many molecular compounds together.
- In an aqueous solution, ions are required for the passage of electricity. When molecular chemicals are mixed with water, they dissolve into molecules rather than ions. Because there are no free mobile ions to conduct electricity, when dissolved in water, they normally do not conduct electricity very effectively.
- A polar solvent, such as water, dissolves polar covalent compounds effectively. Sugar and ethanol are two examples of molecular molecules that dissolve easily in water. Nonpolar covalent compounds, on the other hand, do not dissolve readily in water, such as water and oil. These molecules cannot be hydrated by water.
- Weak intermolecular forces of attraction hold both covalent bonds and organic molecules in organic solvents together. The covalent molecules in covalent compounds are easily miscible with the organic molecules in organic solvents because they have the same sort of weak intermolecular forces of attraction. As a result, most covalent compounds are soluble in organic solvents.
- The bond length is also taken into consideration by Lewis’ theory; the stronger the connection and the more electrons shared, the shorter the bond length.
Difference between Covalent and Ionic Bond
|
Parameters
|
Covalent Bond
|
Ionic Bond
|
|
Definition
|
It is a kind of chemical bonding between two
non-metallic atoms characterized by the sharing
of electron pairs between atoms as well as other
covalent connections. |
It is a sort of connection generated by the strong
the electrostatic force of attraction between
oppositely charged ions in a chemical molecule,
also known as an electrovalent bond. |
|
Occurs between
|
Two non-metals or a non-metal and a metalloid |
One metal and one non-metal |
|
Nature of Component Particles
|
It is made up of discrete electrically neutral molecules. |
Cations and anions are oppositely charged particles
that makeup ionic compounds. |
|
State at Room Temperature
|
Gases, liquids, or low-melting solids |
Crystalline solids |
|
Polarity
|
Low |
High |
|
Solubility
|
In polar fluids, polar covalent molecules dissolve.
HCl in water, for example. Only organic liquids
are soluble in nonpolar covalent compounds. |
Ionic chemicals are only soluble in polar solvents like
water because they are polar. Inorganic liquids are insoluble. |
|
Formation
|
They are created when two non-metals with
identical electronegativities come together.
Neither atom has enough strength to pull
electrons from the other. For stability,
they share electrons from the outermost
orbital with their neighbours. |
They are created when a metal(+ion) and a
non-metal interact (-ve ion). Metals are stronger
than non-metals, and non-metals may easily
get electrons from metals. These two
ions are attracted to one another and
create an ionic connection. |
|
Conductivity
|
Ions do not dissociate from non-polar covalent
molecules. As a result, don’t conduct electricity.
In water, polar covalent compounds quickly
dissociate and act as good electrical conductors.
HCl in water, for example. |
Non-polar covalent compounds do not break down
into ions. As a result, you will not be able to conduct
electricity. Polar covalent compounds break down
readily in water and behave as good electrical
conductors. For instance, HCl in water. |
|
Melting Point
|
Low |
High |
|
Boiling Point
|
Low |
High |
|
Example
|
Methane, Hydrochloric acid |
Sodium Chloride, Sulphuric acid |
Hydrogen Bond
Hydrogen bond is a weaker form of chemical bond than ionic and covalent bonds. It’s a type of polar covalent bond between oxygen and hydrogen in which the hydrogen acquires a partial positive charge. This means that the electrons are being drawn closer to the more electronegative oxygen atom. This causes the hydrogen to be attracted to the negative charges of any neighbouring atom. This type of chemical bonding is known as a hydrogen bond, and it is responsible for many of water’s properties.
Types of Hydrogen Bonding
There are two types of Hydrogen Bonds between or among any molecules. These types are as follows:
- Intermolecular Hydrogen Bond: Those hydrogen bonds that are formed between two different molecules.
- Intramolecular Hydrogen Bond: Those hydrogen bonds that are formed within a molecule.
Metallic Bond
Metallic Bond is the type of chemical bond that forms between metal atoms and gives metals their distinctive properties. Metallic bonds are characterized by the delocalization of electrons in the lattice structure of metals. In metallic bonding, the valence electron of metals are not tightly bounded in the lattice structure thus they can move freely in the entire lattice structure, which emerged as unique properties in metals.
Metallic Bonds give metals their unique properties such as high thermal and electrical conductivity, ductility, malleability, high melting and boiling points, etc. As delocalized electrons can move freely in the lattice structure thus allowing efficient transfer of heat and electricity.
Formation of Metallic Bond
The formation of a metallic bond is explained using the electron sea model. In this model, the metal atoms release their valence electrons and those released valence electrons are shared by all the atoms within the lattice and the positive metal ions are held together by the electrostatic attraction between the cations and the negatively charged sea of delocalized electrons.
The strength of a metallic bond depends on various factors such as the number of valence electrons that are delocalized, the size of metal ions, the arrangement of the metal lattice, etc. Generally, metals with a larger number of valence electrons and a more compact crystal structure tend to have stronger metallic bonds.
Read More,
Sample Questions on Types of Chemical Bonding
Question 1: What is Chemical Bond?
Answer:
Chemical bonding is the process of redistributing electrons between two or more atoms so that each atom achieves a stable electronic state.
Question 2: What are the types of chemical bonds?
Answer:
Covalent, hydrogen bonds, van der Waals contacts, and ionic or electrovalent bonds are the four chemical bonds found in chemistry.
Question 3: Which chemical bonds are the strongest?
Answer:
The covalent link is the most powerful chemical bond. Due to the mutual sharing of electrons, they developed between two atoms. Water is a classic example of a covalent bond because both hydrogen and oxygen atoms exchange electrons.
Question 4: What causes a diamond’s melting point to be so high?
Answer:
Diamond is a carbon allotrope. Each of the carbon atoms in a diamond is covalently bound to four other carbon atoms. As a result, a massive covalent structure is formed. As a result, diamond is very hard and has a very high melting point.
Question 5: What are polar covalent bonds?
Answer:
When electrons are shared unequally, this type of covalent bond forms because the electronegativity of the joining atoms vary. For more electronegative atoms, the pull for electrons will be stronger. The electronegative difference between the atoms is more than zero but less than 2.0. As a result, the shared pair of electrons of that atom will be closer together.
FAQs on Types of Chemical Bonding
Q1: Define Chemical Bonds.
Answer:
Chemical bonds are forces that hold atoms together, forming molecules or compounds through sharing, transferring, or attracting electrons.
Q2: What are Main Types of Chemical Bonds?
Answer:
Chemical bonds are classified into four groups:
- Ionic or Electrovalent Bond
- Covalent Bond
- Hydrogen Bond
- Metallic Bond
Q3: What is an Ionic Bond?
Answer:
Ionic bonds form when transfer of electron happen between different elements or compounds. By transferring an electron to any element, donar element becomes positive and reciver element becomes negetive.
Q4: What is a Covalent Bond?
Answer:
Covalent bonds form when sharing of electron between atoms takes place i.e., atoms obtain a more stable electron configuratiuon by sharing electrons.
Q4: What is Metallic Bonding?
Answer:
Metallic bonding is the type of bonding that occurs in metals. In this type of bonding, the valence electrons are delocalized and free to move throughout the entire metal structure. The positive metal ions are held together by the attraction to the surrounding sea of electrons.
Q5: What determines the Type of Bond that Forms between Atoms?
Answer:
The type of bond that forms between atoms is determined by the difference in electronegativity values of the atoms involved. If the electronegativity difference is large, an ionic bond is more likely to form. If the electronegativity difference is small, a covalent bond is more likely to form.
Q6: Are there Any Compounds that Exhibit both Ionic and Covalent Bonding?
Answer:
Yes, there are compounds that exhibit both ionic and covalent bonding. These compounds are called polar covalent compounds or polar molecules. In such compounds, the electron pairs in the covalent bonds are unequally shared, resulting in a partial positive charge on one atom and a partial negative charge on the other.
Q7: Write an Example of an Ionic Compound.
Answer:
Sodium chloride (NaCl) is a classic example of an ionic compound. Sodium (Na) loses one electron to form a positively charged sodium ion (Na+), while chlorine (Cl) gains one electron to form a negatively charged chloride ion (Cl–). The opposite charges of the ions attract each other, forming an ionic bond.
Q8: Write an Example of a Covalent Compound.
Answer:
Water (H2O) is an example of a covalent compound. Oxygen (O) and hydrogen (H) atoms share electrons to form covalent bonds. Each hydrogen atom shares one electron with the oxygen atom, resulting in two covalent bonds and a stable water molecule.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...