Thermodynamics is a branch of Physics that explains how thermal energy is changed to other forms of energy and the significance of thermal energy in matter. The behavior of heat, work, and temperature, along with their relations to energy and entropy are governed by the Four Laws of Thermodynamics.
Thermodynamics is the study of relations between heat, work, temperature, and energy, focusing on the laws that govern the transformation of energy within a system and its capability to perform work in its environment. These principles delineate how energy shifts within a system and assess the system’s potential to execute beneficial work.
In this article, we will learn about Thermodynamics, Branches of Thermodynamics, Laws of Thermodynamics, Basic Concepts of Thermodynamics, and Examples of Thermodynamics in Daily Life.
What is Thermodynamics?
The term “thermodynamics” is made of two terms, “thermo” and “dynamics” where the term “thermo” refers to heat, and the term “dynamics” refers to a mechanical motion that requires “work.”
So the field of physics that studies the relationship between heat and other types of energy is called thermodynamics.
Creating a clear boundary makes thermodynamics much simpler. The “system” refers to everything contained within the boundary, and the “surroundings” refers to everything outside of it. Once the boundary diagram has been created, the flow across system boundaries can be used to describe the movement and transfer of energy. The word “universe” refers to both the surroundings and the system.
.png)
Thermodynamics Definition
Thermodynamics is a branch of Physics that deals with the concept of energy, heat and temperature and their interrelation with radiation, energy and physical characteristics of matter.
Different Branches of Thermodynamics
The study of Thermodynamics is classified into several branches listed below:
- Classical Thermodynamics
- Statistical Thermodynamics
- Chemical Thermodynamics
- Equilibrium Thermodynamics
- Non-Equilibrium Thermodynamics
Let’s learn about these in detail in the article.
Classical Thermodynamics
The behaviour of matter is examined using a macroscopic perspective in classical thermodynamics. In order to determine the characteristics and predict the characteristics of the matter conducting the process, individuals take into account units like temperature and pressure.
Statistical Thermodynamics
The development of atomic and molecular theories in the late 19th and early 20th centuries gave rise to statistical mechanics, also known as statistical thermodynamics, which added an interpretation of the microscopic interactions between individual particles or quantum-mechanical states to classical thermodynamics. This field explains classical thermodynamics as a natural consequence of statistics, classical mechanics, and quantum theory at the microscopic level. It does this by connecting the microscopic, bulk properties of materials that can be observed on the human scale to the macroscopic, individual atom, and molecule properties.
Chemical Thermodynamics
Chemical thermodynamics is the study of how energy interacts with chemical processes or state changes in accordance with the laws of thermodynamics. Determining the spontaneity of a certain transition is the main goal of chemical thermodynamics.
Equilibrium Thermodynamics
Equilibrium thermodynamics is the study of matter and energy transfers in systems or substances that can be moved from one state of thermodynamic equilibrium to another by agents in their environment. The phrase “thermodynamic equilibrium” refers to a condition of equilibrium in which all macroscopic flows are zero. In the case of the most basic systems or bodies, this means that their intensive properties are uniform and that their pressures are perpendicular to their boundaries. Unbalanced potentials or driving forces between the system’s macroscopically diverse components do not exist in an equilibrium state.
Non-equilibrium Thermodynamics
Systems that are not in thermodynamic equilibrium are the focus of the field of thermodynamics known as non-equilibrium thermodynamics. The majority of systems in nature are not in thermodynamic equilibrium because they are not in stationary states and are subject to fluxes of matter and energy to and from other systems on a continual and irregular basis. More general notions than those covered by equilibrium thermodynamics are needed for the thermodynamic study of non-equilibrium systems.
Basic Concepts of Thermodynamics
There are various concepts in thermodynamics, some of these concepts are explained as follows:
Thermodynamic Systems
A collection of an extremely large number of atoms or molecules confined within certain boundaries such that it has certain values of pressure (P), volume (V) and temperature (T) is called a thermodynamic system.
Anything outside the thermodynamic system to which energy or matter is exchanged is called its surroundings. Taking into consideration the interaction between a system and its surroundings, a system said to be an open system if it can exchange both energy and matter with its surroundings may be divided into three classes-
- Open system: A system is said to be an open system if it can exchange energy and matter with its surroundings.
- Closed system: A system is said to be a closed system if it can exchange only energy (not matter) with its surroundings.
- Isolated system: A system is said to be isolated if it can neither exchange energy nor matter with its surroundings.
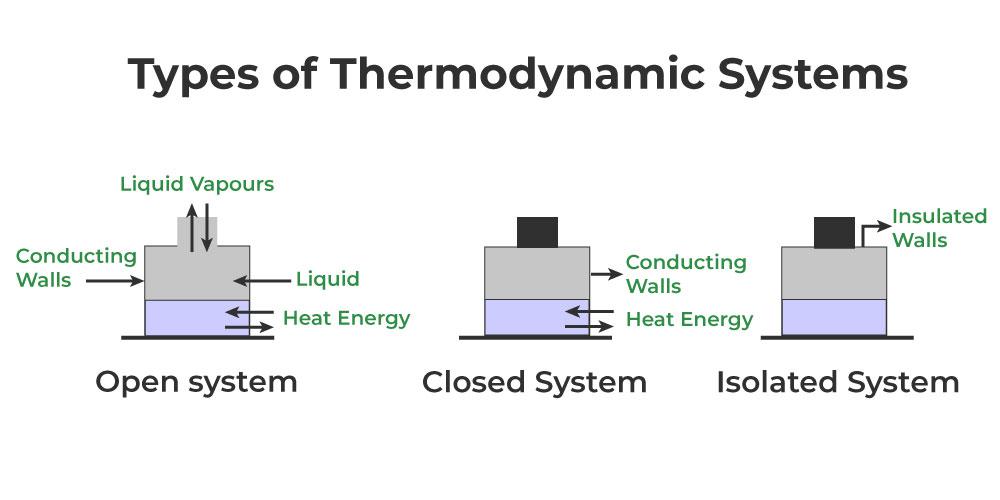
Type of Systems
Surroundings
The term “surroundings” refers to everything outside the system that affects how it behaves. There is a boundary separating the system from its surroundings. It could be stationary, mobile, or fictitious. It won’t take up any space in terms of mass or volume. For example, consider a closed beaker with liquid inside as shown below. The liquid inside the beaker is the system, while the outline of the beaker represents the boundary of the system. And matter outside the system and boundary is called its surroundings.

System and Surroundings
Heat
Heat is energy that is transmitted between objects or systems as a result of a temperature difference. Heat is conserved energy, which means it cannot be created or destroyed. However, it can be moved from one location to another. Additionally, heat can be transformed into and out of various types of energy.
Work
The work done by a system or on a system during a process depends not only on the system’s starting and final states but also on the path chosen for the process. When a force acting on a system moves the body in its own direction, work has been done. Force and displacement combine to create the work (W) that is done to or by a system.
Internal Energy
The kinetic and potential energies of the molecules are added up to form internal energy. The system’s internal energy is represented by the letter U. Kinetic energy is the energy that molecules or atoms possess due to their motion. Two molecules have some potential energy because they are attracted to one another. The total kinetic and potential energy of the atoms or molecules that make up a system is what is known as the system’s internal energy.
Thermodynamic Properties or Variables
A thermodynamic system can be described by specifying its pressure, volume, temperature, internal energy, enthalpy, and the number of moles. These parameters or variables are called thermodynamic variables.
Thus, the variables which are required to specify the state of the thermodynamic system are called thermodynamic variables. Entropy is a measurement of energy present in a system or process but is not available to do work. It is also defined as the measure of disorder in the system. Enthalpy is the measurement of the total energy of a thermodynamic system.
Types of Thermodynamic Variables
- Intensive variables: The variables which are independent of the size of the system are called intensive variables. e.g. Temperature, pressure, and specific beat capacity.
- Extensive variables: The variables which depend on the size or mass of the system are called extensive variables. e.g. Volume, energy, entropy, heat capacity, and enthalpy.
Thermodynamic Equilibrium
Thermodynamic equilibrium is a state of a system in which there is no net change occurs within the system over time i.e., the state of a thermodynamic system in which macroscopic properties such as temperature, pressure, and chemical composition, remain constant. There are different types of thermodynamic equilibrium:
- Thermal Equilibrium: In the state of thermal equilibrium, the temperature of the system remains constant i.e., there is no net heat transfer occurring within the system.
- Mechanical Equilibrium: When in a system either there is no net force acting or there is no pressure difference, then this state of the system is called mechanical equilibrium. This state is also sometimes referred to as a state of mechanical balance.
- Chemical Equilibrium: A thermodynamic system is said to be in chemical equilibrium when the rates of forward and reverse reactions become equal. When the system reaches chemical equilibrium, the concentrations of reactants and products remain constant over time.
- Phase Equilibrium: A system is said to be in Phase equilibrium when different phases of a substance such as solid, liquid, and gas, coexist in a system at the same time. In other words, the system is in phase equilibrium when the rates of phase changes, such as evaporation and condensation, are equal.
Thermal Equilibrium
When two bodies having different temperatures are placed in contact, then the energy flows from a body at a higher temperature to a body at a lower temperature. The flow of energy continues from one body to another to attain the same temperature.
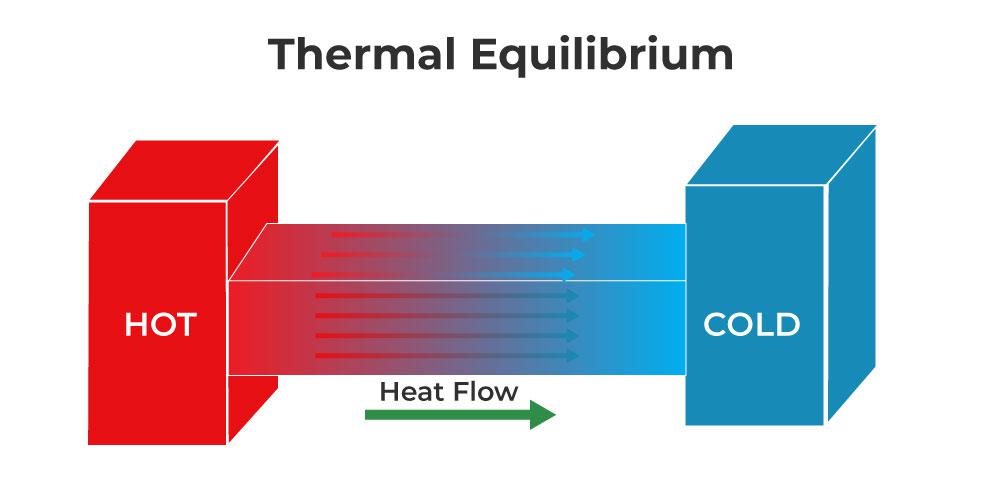
thermal Equilibrium
When both the bodies in contact have the same temperature and there is no energy for the body till both bodies are between them, then these bodies are in thermal equilibrium. Thus, two bodies or systems in contact are said to be in thermal equilibrium if both are at the same temperature.
- A thermodynamic system is said to be in thermodynamic equilibrium if its variables like pressure, volume, temperature, number of particles, etc. do not change with time.
- Any isolated system is in thermodynamic equilibrium.
Thermodynamic Processes
Any process in which the thermodynamic variables of a thermodynamic system change is known as the thermodynamic process.
- Quasi-Static Process (Quasi-Static means nearly static): A process in which the system departs only infinitesimally from the equilibrium state is known as a quasi-static process In this process, the change in pressure or change in volume or change in temperature of the system is very very small.
- Isothermal Process: A process in which the pressure and volume of the system change at constant temperature are called the isothermal process. In this case, P and V change but T is constant. i.e. dT (change in temperature) = 0.
- Adiabatic Process: A process in which pressure, volume, and temperature of the system change, but there is no exchange of heat between the and its surroundings is called the adiabatic process. In this case, P. V and T change but Q = 0. The system should be compressed or allowed to expand suddenly so that there is no time for the exchange of heat between the system and its surroundings. Since these two conditions are not fully realized in practice, so no process is perfectly adiabatic.
- Isochoric Process: A thermodynamic process that takes place at constant volume is called the isochoric process. It is also known as the isovolumic process. In this case, dV = 0.
- Isobaric Process: A thermodynamic process that takes place at constant pressure is called the isobaric process. In this case, dP = 0.
- Cyclic Process: A cyclic process consists of a series of changes that return the system to its initial state.
Thermodynamic Potentials
The stored energy in a system is measured by its thermodynamic potentials. Potentials measure how a system’s energy transforms from its initial state to its final one. Depending on the constraints of the system, such as temperature and pressure, different potentials are used.
Different forms of thermodynamic potentials are mentioned below:
- Internal Energy (U): It is equal to the sum of the ability to do work and the ability to release heat.
- Gibbs Energy (G): It is the ability to do non-mechanical work.
- Enthalpy (H): It is the ability to do non-mechanical work and the ability to emit heat.
- Helmholtz Energy (F): It is the ability to do both mechanical and non-mechanical work.
What is Enthalpy?
In a thermodynamic system, energy is measured by enthalpy. Enthalpy is a measure of a system’s total heat content and is equal to the system’s internal energy plus the sum of its volume and pressure. Enthalpy is a property or state function that resembles energy; it has the same dimensions as energy and is therefore measured in joules or ergs. The value of enthalpy is entirely dependent on the temperature, pressure, and composition of the system, not on its history.
What is Entropy in Thermodynamics?
Entropy is the measurement of the amount of thermal energy per unit of temperature in a system that cannot be used for useful work. Entropy is a measure of a system’s molecular disorder or randomness since work is produced by ordered molecular motion. Entropy theory offers a deep understanding of the direction of spontaneous change for many common events.
Laws of Thermodynamics
Thermodynamic systems at thermal equilibrium are characterized by fundamental physical constants like energy, temperature, and entropy, which are defined by thermodynamic laws. These thermodynamic principles describe how these quantities act in different situations.
How many laws of thermodynamics are there?
There are four laws of thermodynamics which are listed as follows:
- Zeroth Law of Thermodynamics
- First Law of Thermodynamics
- Second Law of Thermodynamics
- Third Law of Thermodynamics
Zeroth Law of Thermodynamics
According to the Zeroth Law of Thermodynamics, if two bodies are separately in thermal equilibrium with a third body, then the first two bodies are likewise in thermal equilibrium with each other. This indicates that if system A is in thermal equilibrium with system B, and system C is likewise in thermal equilibrium with system B, then both systems A and C are in thermal equilibrium.
Example showing Zeroth Law of Thermodynamics:
Imagine you have three containers of water, A, B, and C. Container A is at a temperature of 50°C, container B is at 60°C, and container C is at 70°C. Now, we want to determine if containers A, B, and C are in thermal equilibrium with each other based on the Zeroth Law.
Step 1: Confirm that A and C are in thermal equilibrium with the third system (B). To do this, we bring container B into contact with container A and check if no heat transfer occurs. After some time, no heat flows between A and B, indicating they have reached thermal equilibrium. Similarly, if we bring container B into contact with container C and find no heat transfer between them, it implies that C and B are also in thermal equilibrium.
Step 2: Now, we can conclude that A and C are in thermal equilibrium because they both meet the condition of being in thermal equilibrium with B. Therefore, by the Zeroth Law, A and C are in thermal equilibrium with each other as well.
So, based on the Zeroth Law, we can say that containers A, B, and C are all in thermal equilibrium with each other. This means that their temperatures can be considered equal on the same temperature scale, and we can measure temperature differences and changes accordingly.
First Law of Thermodynamics
Energy cannot be generated or destroyed, according to the first law of thermodynamics, but it can be converted from one form to another. Heat, internal energy, and work are all addressed by the first law of thermodynamics. Energy cannot be generated or destroyed, according to the first law of thermodynamics, but it can be converted from one form to another. According to this law, some of the heat provided to the system is utilized to change the internal energy, while the remaining is used to perform work. First law of thermodynamics is also known as the law of conservation of energy.

Mathematical Form of First Law of Thermodynamics:
Mathematically, it may be expressed as
ΔQ = ΔU + W
Where,
The heat given or lost is denoted by ΔQ.
The change in internal energy is denoted by ΔU.
W stands for work done.
The above equation can alternatively be written as follows:
ΔU = ΔQ − W
As a result of the above equation, we may deduce that the quantity (ΔQ – W) is unaffected by the path taken to change the state. Furthermore, when heat is applied to a system, internal energy tends to rise, and vice versa.
Read in Detail:
Second Law of Thermodynamics
In an isolated system, the second law of thermodynamics asserts that entropy always increases. Any isolated system progresses spontaneously toward thermal equilibrium or the state of maximum entropy. The universe’s entropy is always increasing and never decreasing.

Read in Detail: Second Law of Thermodynamics
Third Law of Thermodynamics
The third law of thermodynamics states that when the temperature approaches absolute zero, the entropy of a system approaches a constant value. At absolute zero temperature, the entropy of a pure crystalline solid is zero. If the perfect crystal has only one state with minimum energy, this assertion holds true.

Thermodynamics Examples in Daily Life
We came across various examples in our daily life which can be explained using thermodynamic properties. Some of them are,
- Melting of Ice Cubes: Drinks with ice cubes become cooler as the heat from the drink is absorbed. If we neglect to drink it, it will eventually warm back up to room temperature by absorbing heat from the environment. The first and second laws of thermodynamics govern how all of this works.
- Sweating in a Crowded Room: In a crowded room, everyone begins to sweat. By transmitting body heat to the sweating, the body begins to cool down. Sweat evaporates, heating the space. Again, this occurs as a result of the application of the first and second laws of thermodynamics. Keep in mind that heat is not lost but rather moved until equilibrium is reached with the least amount of entropy.
- Flipping a Light Switch: Different kinds of power plants, including thermal and nuclear ones, are studied using thermodynamics.
Summary – Thermodynamics
Thermodynamics is a critical branch of physics that studies the movement and transformation of thermal energy into other forms of energy, highlighting its importance in the behavior of matter. This field delves into the intricate relationships between heat, work, temperature, and energy, governed by the Four Laws of Thermodynamics. These laws outline the principles of energy conversion within a system, its interaction with the surroundings, and its ability to perform useful work.
Thermodynamics Examples
Example 1: Calculate ΔG at 280 K for the reaction,
2NO + O2 → 2NO2
when ΔH and ΔS of the reaction are -100 J and -0.25 J/K respectively
Solution:
Given,
ΔH = -100 J
ΔS = -0.25 J/K
T = 280 K
We know that,
ΔG = ΔH – TΔS
⇒ ΔG = (-100) -280(-0.25)
⇒ ΔG = -100 + 70
⇒ ΔG = -30 J
Example 2: Calculate the temperature at which ΔG of the given reaction is 200 J when ΔH and ΔS of the reaction are -150 J and -0.5 J/K respectively
H2 + I2 → 2HI
Solution:
Given,
ΔG = 200 J
ΔH = -150 J
ΔS = -0.5 J/K
T =?
We know that,
ΔG = ΔH – TΔS
Thus,
⇒ 200 = (-150) – T(-0.5)
⇒ 200 = -150 + T/2
⇒ 200 + 150 = T/2
⇒ T = 700 K
Example 3: If an ideal heat engine operates in a Carnot cycle between 600 K and 400 K and if it absorbs 6000 J of heat at a higher temperature then find the heat supplied from the source.
Solution:
Given,
T1 = 600 K
T2 = 400 K
Heat Absorbed at High Temperature = 6000 J
Heat Supplied from Source =?
Efficiency of Heat Engine (E) = 1 – (T2 / T1)
⇒ Efficiency of Heat Engine (E) = 1 – 400/600 = 1- 2/3
⇒ Efficiency of Heat Engine (E) = 1/3
We know that,
E = Heat Supplied from Source/Heat Absorbed at High Temperature
⇒1/3 = Heat Supplied from Source/6000
Heat Supplied from Source = 2000 J
Example 4: Find the Efficiency of the Heat Engine if it operates between 700 K and 350 K.
Solution:
T1 = 700 K
T2 = 350 K
Efficiency of Heat Engine (E) = 1 – (T2 / T1)
⇒ Efficiency of Heat Engine (E) = 1 – 350/700 = 1- 1/2
⇒ Efficiency of Heat Engine (E) = 1/2
⇒ E = 1/2 × 100 % = 50%
Thermodynamics Class 11
In Class 11, thermodynamics is introduced as a foundational topic in physics (and also in chemistry), focusing on the principles that govern the behavior of heat and energy. The curriculum is designed to provide students with a solid understanding of the basic concepts, laws, and applications of thermodynamics.
The thermodynamics section in Class 11 is critical for building a strong foundation for further study in various fields of science and engineering. It provides the basic vocabulary and conceptual framework that students need to tackle more complex problems and to understand the physical world around them.
Thermodynamics JEE Mains Questions
Q1. 28.0 L of CO2 is produced on complete combustion of 16.8 L gaseous mixture of Ethene and methane at 25 ℃ and 1 atm. Heat evolved during combustion process is ___ kJ. Given: ΔHc(CH4) = -900 kjmol-1, ΔHc(CH4) = -1400 kjmol-1
Q2. When 2 litre of Ideal gas expands isothermally into vacuum to total of 6 litre, the change in internal energy is ___J. (Nearest Integers).
Q3. 1 mole of Ideal gas is allowed to expands reversibly and adiabatically from a temperature of 27 ℃. the workdone is 3 kJmol-1. The final temperature of the gas is ___ K (Nearest Integer). Given Cv = 20 J mol-1K-1.
Q4. Enthalpies of formation of CCl4(g), H2O(g), CO2(g) and HCl(g) are -105, -242, -394, and -92 kJmol-1 respectively. The magnitude of enthalpy of the reaction given below is kJmol-1. (Nearest Integer)
CCl4(g) + 2H2O(g) –> CO2(g) + 4HCl(g)
Q5. The value of log K for the reaction A ⇋ B at 298 K is —–(Nearest Integers). Given: ΔH° = -54.07 kJmol-1, ΔS° = 10 JK-1mol-1.
FAQs on Thermodynamics
What is an Isolated System?
A system is said to be isolated if it can neither exchange energy nor matter with its surroundings.
What is the significance of Thermodynamics Laws?
Temperature, energy, and entropy, which describe the physical quantities that define thermodynamic systems at thermal equilibrium, are defined by the laws of thermodynamics.
Can energy be lost or destroyed?
No, the energy can not be destroyed and created. But it can only be transferred from one form of energy to other.
What does Negative Work look like?
An example of negative work is when you’re pushing an object along the floor, the work done by Kinetic Friction is negative.
Do the Laws of Thermodynamics apply to the human body?
The human body really does follow the law of thermodynamics. You start to feel warm and begin to sweat when you are in a crowded room with other people. This is how the body cools itself.
What is the Zeroth Law of Thermodynamics?
The Zeroth Law of Thermodynamics states “If two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.”
What is the First Law of Thermodynamics?
The First Law of Thermodynamics, also known as the Law of Energy Conservation, states “The total energy of an isolated system is conserved; energy can neither be created nor destroyed, only converted from one form to another.”
What is the Second Law of Thermodynamics?
The Second Law of Thermodynamics states that “It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a single reservoir to a single sink while doing net work.”
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...