Reactivity Series – Reactivity of Metals, Features, Tricks
Last Updated :
24 Apr, 2024
Reactivity Series is an arrangement of metals from highest to lowest reactivity order. Therefore, the term “reactivity series” refers to a series of metals arranged in descending order of reactivity. It is also known by the name “activity series.”

Reactivity Series
Let’s understand about Reactivity Series, reactivity series table in detail below.
Reactivity Series Definition
The arrangement of metals in a series of decreasing order of their tendencies to react is called the Reactivity Series of metal. Therefore, the least reactive metal (Platinum) is placed at the bottom while, the highly reactive metal (Potassium) is at the top of this series.
It has been observed that some metals react quickly and strongly with reactants, while others react slowly, or some do not react at all. This is because of the reactivity of the metal. Here, reactivity means the ability of a chemical substance to give the product on its own.
Reactivity Series Table
The Reactivity series of some of the most common metals, arranged in descending order of reactivity which can be given as follows:
|
Metal
|
Ion
|
Reactivity
|
| Potassium |
K+ |
React with Cold Water
|
| Sodium |
Na+ |
| Lithium |
Li+ |
| Barium |
Ba+ |
| Strontium |
Sr+ |
| Calcium |
Ca+ |
| Magnesium |
Mg+ |
Reacts very slowly with cold water,
whereas quickly in boiling water,
and very strongly with acids.
|
| Beryllium |
Be2+ |
Reacts with steam and acids
|
| Aluminum |
Al3+ |
| Titanium |
Ti4+ |
Reacts with concentrated mineral acids
|
| Manganese |
Mn2+ |
Reacts with acids
|
| Zinc |
Zn2+ |
| Chromium |
Cr3+ |
| Iron |
Fe2+ |
| Cadmium |
Cd2+ |
| Cobalt |
Co2+ |
| Nickel |
Ni2+ |
| Tin |
Sn2+ |
| Lead |
Pb2+ |
| Antimony |
Sb3+ |
May react with some strong
oxidizing acids
|
| Bismuth |
Bi3+ |
| Copper |
Cu2+ |
React slowly with water
|
| Tungsten |
W3+ |
Highly unreactive May react with
some of the strong oxidizing acids
|
| Mercury |
Hg2+ |
| Silver |
Ag+ |
| Gold |
Au3+ |
Reactivity Series of Metals
To determine if a metal can displace another in a single displacement reaction, the data provided by the reactivity series can be utilized. It can also be used to find out how reactive various metals are to acids and water.
Below is a chart showing the reactivity series of common metals:
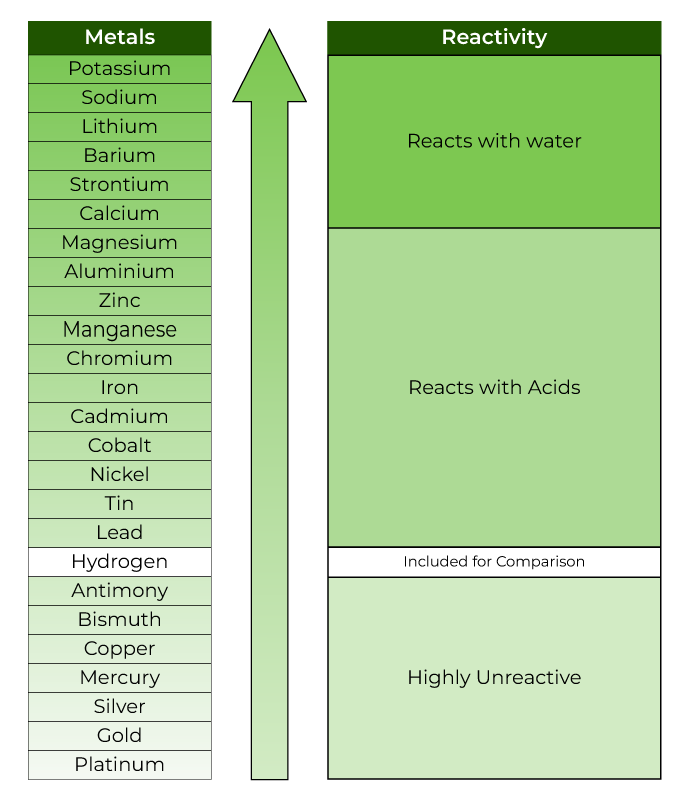
Properties of Reactivity Series
- The reducing tendency of metals at the top of the table has high, which is why they are easily oxidized. These metals can get tarnished or corrode very easily. For example, sodium due to its high reactivity explodes as came in contact with air or water, thus needs to be kept away from moisture and air, and dipped in kerosene.
- The electro-positivity (tendency to lose electrons) of the elements gets reduced while moving down the reactivity series of metals, as we go down in the series it’s hard for elements to lose electrons, hence the less reactivity.
- The tendency to reduce the metals becomes weaker while traversing down the series.
- On reaction with dilute HCl or dilute H2SO4, all metals that are found above hydrogen in the activity series liberate H2 gas and form salt.
- Higher-ranking metals require greater amounts of energy for their isolation from ores and other compounds.
Uses of Reactivity Series
The reactivity series provides the study of properties and reactivities of the metals, Apart from this reactivity series also provides several other important applications. For example, the result we get out of the reactions between metals and acids, metals and water, and single displacement reactions between metals can be predicted.
Reaction Between Metals and Water
Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the corresponding hydroxide while liberating hydrogen gas. For example, the reaction between potassium and water yields potassium hydroxide and H2 gas, as described by the chemical equation provided below.
2K + 2H2O → 2KOH + H2
Single Displacement Reactions between Metals
Highly reactive metals can reduce the less reactive metals from their salts in an aqueous solution and this happens through a single displacement reaction. For example, copper can easily displace silver from the silver nitrate solution, as copper is much more reactive than silver and the balanced chemical reaction of the same is given as follows:
Cu(s) + 2AgNO3 (aq) → Cu(NO3)2(aq) + 2Ag(s)
Reaction with Acid and Base
Metals that are more reactive than hydrogen can displace hydrogen from dilute acids, and form respective metal salts and hydrogen gas.
Metal + Dilute Acid or Base → Salt + Hydrogen gas
For example, sodium metal has a tendency to displace hydrogen from hydrogen chloride (HCl) to form sodium chloride and liberate hydrogen gas. The reaction is given below:
2Na(s) + 2HCl (dilute) → 2NaCl (aq) + H2(g)
For another example, Iron can displace hydrogen from the sulfuric acid to make Iron Sulfate and Hydrogen gas, and the balanced chemical reaction of the same is as follows:
Fe(s) + H2SO4 (dilute) → FeSO4(aq) + H2(g)
For another example for Base, When zinc reacts with sodium hydroxide, it gives an aqueous solution of sodium zincate (as zinc can’t replace sodium from the base due to less reactivity) and hydrogen gas. The balance chemical reaction of the same is as follows:
Zn(s) + 2NaOH (aq) → Na2ZnO2(aq) + H2(g)
Reactivity Series Trick
The basic strategy for learning the reactivity series is to group these elements into meaningful groups and then use a mnemonic for each group.
Here we have used the sentence “Please send charlie’s monkeys and zebras in lead & hydrogen cages in mountains securely guarded by Plato.” The first letter of each word represents a metal of the reactivity in the order from highest to lowest as:
|
Symbol
|
Metal of the
Reactivity Series
|
Words/Mnemonic used to
Remember the Series
|
|
K
|
Potassium
|
Please
|
|
Na
|
Sodium
|
Send
|
|
Ca
|
Calcium
|
Charlie’s
|
|
Mg
|
Magnesium
|
Monkey
|
|
Al
|
Aluminium
|
And
|
|
Zn
|
Zinc
|
Zebra
|
|
Fe
|
Iron
|
In
|
|
Pb
|
Lead
|
Lead &
|
|
H
|
Hydrogen
|
Hydrogen
|
|
Cu
|
Copper
|
Cages in
|
|
Hg
|
Mercury
|
Mountains
|
|
Ag
|
Silver
|
Securely
|
|
Au
|
Gold
|
Guarded by
|
|
Pt
|
Platinum
|
Plato
|
Related
Reactivity Series – FAQs
What is Reactivity Series of Metals?
The arrangement of metals in a series of decreasing order of their tendencies to react is called the Reactivity Series of metal.
What does Reactivity Series show?
Reactivity series shows a list of metals arranged based on their tendencies to react from highest to lowest order. This implies that the most reactive metals are at the top while the least reactive metals at the bottom.
Which is the Most Reactive Metal?
According to the reactivity series of metals, Potassium (K) is the highly reactive metal.
Which Metal is the Least Reactive?
According to the reactivity series of metals, Platinum (Pt) is the least reactive metal.
What does Reactivity Series of Metals depend on?
The reactivity of metals is determined by its reactivity, which is influenced by the atomic radius, nuclear charge, arrangement of the sublevel electrons, and shielding effect.
Why is Hydrogen placed in the Reactivity Series of Metal?
Hydrogen is placed in the reactivity series of metals because it can exhibit metallic properties under certain conditions, such as forming positive ions (H⁺) in reactions, similar to other metals.
Are Non Metals Reactive or Non Reactive?
Non-metals can be both reactive and non-reactive, depending on the specific element and its conditions. Some non-metals, like fluorine and oxygen, are highly reactive and readily participate in chemical reactions. Others, such as noble gases like helium and neon, are generally non-reactive under normal conditions.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...