Organic chemistry is a branch of science that studies the structure, properties, and interactions of organic compounds having covalent carbon bonds. By examining their structure, their structural formula can be derived. To better understand their behavior, physical and chemical properties, as well as chemical reactivity, are investigated. The study of organic processes includes the chemical synthesis of natural products, pharmaceuticals, and polymers, as well as laboratory and theoretical (in silico) studies of individual organic molecules.
Organic chemistry studies hydrocarbons (compounds comprising only carbon and hydrogen) and compounds based on carbon but also includes other elements such as oxygen, nitrogen, sulphur, phosphorus (present in many biochemicals), and the halogens. The study of compounds containing organometallic elements is known as organometallic chemistry.
Qualitative Analysis of Organic Compounds
After organic compounds have been synthesized in their purest form, qualitative analysis is used to identify their structure and characterization. The analysis provides insight into the constituents that make up the chemical. Carbon, hydrogen, oxygen, and nitrogen (in some cases) are the most prevalent components found in organic molecules, hence they are detected through analysis. Sulfur, halogens, metals, and phosphorus are occasionally found and detected via qualitative analysis.
Detection of Carbon and Hydrogen
Principle
Dry copper (II) oxide or cupric oxide can be used to detect carbon and hydrogen in an organic molecule. In a hard glass tube, the organic compound is heated with dry copper (II) oxide. The compound’s carbon will be oxidized to carbon dioxide, while the hydrogen will be oxidized to water. The following are the reactions:
C + 2CuOΔ → CO2 + 2Cu
2H + 2CuOΔ → HO2 + 2Cu
For example, if the organic chemical under test has the molecular formula CxHy, then its full combustion in the presence of cupric oxide can be stated as:
CxHy + (2x + y/2)CuOΔ → xCO2 + y/2H2O + (2x + y/2)Cu
The ability of carbon dioxide to render lime water milky can be used to detect it (Calcium Carbonate is formed). The ability of water to condense on the cool sections of the test tube can be used to identify it. Anhydrous copper sulfate becomes ‘blue’ as a result of this reaction.
Ca(OH)2 + CO2 → CaCO3 + H2O
CuSO4 + 5H2O → CuSO4 ⋅ 5H2O
Procedure
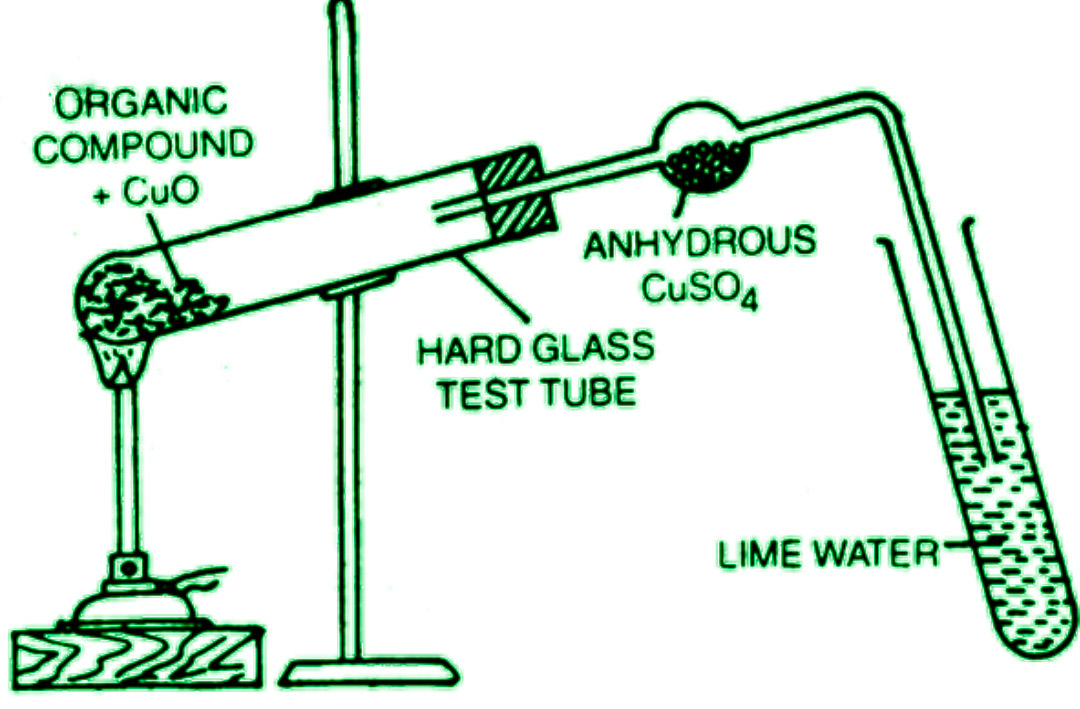
A small amount of the dry and pure organic component is mixed with about 5-6 times the weight of dry and pure cupric oxide powder in the laboratory. The combination is then heated to a high temperature in a rigid test tube with a delivery tube. The delivery tube has a bulb in the middle and is dipped into the lime water on the other end, as depicted.
Glass wool and anhydrous copper sulfate are put into the delivery tube’s bulb. When carbon is heated, it is oxidized to carbon dioxide, turning the lime water milky. The anhydrous copper sulfate on the bulb becomes blue when hydrogen in the organic chemical is oxidized to water, indicating its presence.
Detection of Nitrogen
Nitrogen is found in some organic molecules, such as amines and nitro compounds. A set of experiments are used to detect nitrogen in various organic molecules, as follows:
- Dry heating test: It confirms the presence of nitrogen when the organic component under examination is intensely heated and generates a burnt hair or feather odor. However, the test has limits because many nitrogen-containing substances do not generate odor.
- Soda-lime test: In a dry test tube, a little amount of organic substance is heated vigorously with soda lime (NaOH+CaO). The presence of nitrogen is indicated if the vapors smell like ammonia.
NH2CONH2 + 2NaOHCaO,Δ → 2NH3 + Na2CO3
However, these assays have limitations, like many organic compounds with Nitro and Azo (–N=N–) groups do not show up in this test.
- Lassaigne’s test: Lassaigne’s test is used to detect nitrogen, halogens, and sulfur in an organic compound. This is one of the most reliable tests used to detect these components. The elements present in the organic compounds are fused with sodium metal to convert them from covalent to their ionic form.
- Preparing Lassaigne’s extract: In a fusion tube, a little amount of sodium is heated vigorously until it forms a globule. The tube is removed from the flame once the globule has formed, and a little amount of organic chemical is added to the tube, which is then heated vigorously until the tube turns red hot. Meanwhile, prepare a China dish with 10-15 mL of water. When the tube becomes red hot, it is dipped into a water-filled China dish and filtered. The resulting filtrate is known as the Sodium Fusion Extract or Lassaigne’s Extract.
- Testing for Nitrogen element: Because the sodium in the extract combines with the excess water to generate sodium hydroxide, the sodium fusion extract is alkaline. To keep the solution alkaline, sodium hydroxide is sometimes added. To this alkaline solution of Sodium fusion extract, a freshly made ferrous sulfate (FeSO4) is added. The mixture is then slightly warmed before being chilled. To acidify the solution, a small amount of dilute sulfuric acid is added. If there is nitrogen in the solution, it will turn green or blue. The presence of nitrogen and sulfur in the organic component is indicated by the solution turning blood-red.
The reactions for the above test are as follows:
During the fusion process, the carbon and nitrogen in the organic component combine to generate sodium cyanide, as shown:
Na + C + N → NaCN
When sodium ferrocyanide or sodium hexacyanoferrate (II) is heated with ferrous sulfate solution, some of the Fe2+ or ferrous ions are oxidized to ferric (Fe3+) ions. Prussian blue is produced when ferric ions combine with sodium hexacyanoferrate (II) to yield Iron (II) hexacyanoferrate (II) or ferric ferrocyanide.
- 2NaCN + FeSO4 → Na2SO4 + Fe(CN)2
- Fe(CN)2 + 4NaCN → Na4[Fe(CN)6] Sodiumhexacyanoferrate (ii)
- 3Na4[Fe(CN)6] + 4Fe3+ → Fe4[Fe(CN)6]3
Detection of Halogens
The halogens, if present in the organic compound, can be detected using the following tests:
- Beilstein’s Test: Beilstein’s test for detecting halogens in organic compounds is a simple, sensitive, and effective method. A clean and stout copper wire is heated in the Bunsen burner’s non-luminous flame until the flame’s bluish-green or greenish color fades. After that, the wire is immersed in the organic compound and exposed to the flame once more. The creation of cupric halides, and hence the presence of halogen in the compound, is indicated by the flame turning bluish-green or green. Even molecules like urea and thiourea, for example, display this test due to the creation of volatile cupric cyanide, which has some restrictions. It also doesn’t say what kind of halogens are present in the chemical (bromine, chlorine, or iodine).
- Lassaigne’s Test: It is an extremely accurate assay for detecting halogens in organic compounds. The preparation of Sodium Fusion Extract or Lassaigne’s Extract is the first stage. The halogens in organic compounds are transformed to sodium halides in the following way:
Na + X → NaX
The next step is to boil a portion of the extract in weak nitric acid and then strain the solution. A few drops of silver nitrate solution are added to this and the following precipitates are tested:
- Chlorine is indicated by the formation of a white precipitate that is soluble in ammonia but insoluble in dilute HNO3.
- The presence of bromine is indicated by the formation of a pale-yellow precipitate that is somewhat soluble in ammonia.
- The presence of iodine is indicated by the formation of a yellow precipitate that is insoluble in ammonia.
Detection of Sulphur
Sulfur, present in an organic compound can be detected using the following tests:
- Lassaigne’s Test: When a sulfur-containing organic component is fused with sodium, the resulting extract contains sodium sulfide.
2Na + S → Na2S
The presence of sulfur in the chemical will be confirmed by the following tests:
- Sodium Nitroprusside Test: If a small amount of sodium fusion extract is treated with a few drops of sodium nitroprusside, the compound will turn violet, indicating that it contains sulfur. Standing still causes the violet color to fade slowly.
Na2S + Na4[Fe(CN)5(NO)] → Na4[Fe(CN)5(NOS)]
- Lead Acetate Test: Dilute acetic acid is added to the second half of the portion to acidify it, followed by a few drops of lead acetate. The presence of sulfur in the organic compound is established if a dark precipitate indicating the development of lead sulfide is observed.
Na2 S + (CH3COO)2 Pb → PbS + 2CH3COONa
Detection of Phosphorus
By fusing the organic compound with sodium peroxide, an oxidizing agent, the presence of phosphorus in the organic component can be determined. The organic compound’s phosphorus is oxidized to sodium phosphate.
5Na2O2 + 2PΔ → 2Na3PO4 + 2Na2O
Water is used to remove the fused material, and the resulting aqueous solution is subsequently heated with strong nitric acid. Ammonium molybdate is added to this. The presence of phosphorus is indicated by a yellow precipitate or color (due to the production of ammonium phosphomolybdate).
Detection of Oxygen
While there are no direct tests for detecting the presence of oxygen in an organic substance, the procedures listed below can establish its existence indirectly
- The presence of functional groups or tests to confirm the presence of functional groups such as −OH,−COOH,−NO2, etc., indicates the presence of oxygen indirectly.
- When the sum percentages of components in an organic molecule determined by quantitative analysis do not equal 100 %, the differential sum indicates the presence of oxygen, and its proportion can be calculated.
Sample Problems
Question 1: What is qualitative analysis for organic compounds and why it is important?
Answer
Organic compound qualitative analysis is a non-quantifiable analysis of organic compounds. It’s significant since it provides a detailed account of the elements found in organic molecules.
Question 2: Which steps are used in the qualitative analysis of organic compounds?
Answer
For different elements such as oxygen, nitrogen, carbon and hydrogen, sulphur, phosphorus, and halogens, qualitative analysis employs various methods of detection or tests. Each of these tests has its own set of instructions to follow and must be completed independently.
Question 3: Which method is used for the detection of Nitrogen in an organic compound?
Answer
Three separate tests are used to detect nitrogen: the dry heating test, the soda lime test, and Lassaigne’s test.
Question 4: How can we detect the presence of sulfur in an organic compound?
Answer
Sulphur can be detected by utilising Lassaigne’s extract and treating it with either sodium nitroprusside or lead acetate and acetic acid to produce a violet coloration or a yellow precipitate or coloration.
Question 5: What are the types of qualitative analysis?
Answer
Individual constituents in an organic complex can be detected through qualitative analysis. Each element has its own set of tests that are used to validate that it is present in the organic complex under examination.
Question 6: How oxygen is being detected?
Answer
- The existence of functional groups or tests to validate the presence of functional groups (such as OH, COOH, NO2, etc.) indirectly implies the presence of oxygen.
- The differential sum reveals the existence of oxygen, and its proportion may be computed, when the total percentages of components in an organic molecule determined by quantitative analysis do not equal 100%.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...