Prove That Liquids have No fixed Shape but have a Fixed Volume
Last Updated :
17 Aug, 2021
The liquid state of matter has a fixed size, in terms of the occupied volume. The state of matter occupies constant space or volume. A liquid contains a fixed number of molecules, irrespective of the container it is placed in, in case there is no addition or deletion of the liquid in the container.
Liquids occupy a fixed volume but no fixed shape. This is because the particles in the liquid state are not packed closely and also arranged disorderly. There are spaces between the particles, that is, the particles are bound by loose intra-molecular forces of attraction. The particles can move together in a disorganized orientation with respect to the solid-state of matter.
The liquid takes the shape of the container in which it is placed, owing to the weak forces of attraction between the molecules. Therefore, the liquids can change shape continuously.
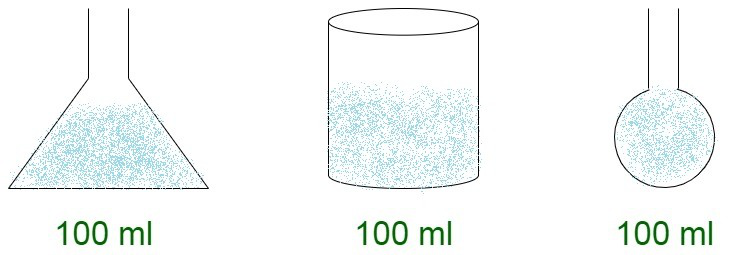
Experiment to show that liquids don’t have a fixed shape but a fixed volume
Step 1: Take a fixed quantity of liquid in a container, say a beaker.
Step 2: For instance, take 100ml of water and pour it into a beaker.
Step 3: Now, making sure, there is no water added or deleted into the container, transfer the water to another container, like a flask with markings of quantities.
Step 4: The water will measure 100ml.
This experiment shows that the volume of water remains constant irrespective of the shape of the container.
Sample Questions
Question 1: Why are liquids termed as incompressible?
Answer:
Upon application of pressure, the volume of liquids remains constant. Other states of matter, for example, gases, change their volume upon application of pressure. Therefore, they are termed as incompressible.
Question 2: Explain some characteristic properties of the liquid state.
Answer:
- The particles are bound together by intramolecular forces of attraction.
- Particles occupy intra-molecular space.
- The particles are in a state of continuous random motion.
Question 3: You apply a pressure of 100mm on one container containing 1L water and 200mm on another container containing 1L water. Which will have lower volume?
Answer:
Both the containers will have same volume equivalent to 1L, since liquids are incompressible.
Question 4: Why do liquids have constant volume?
Answer:
Liquids display strong intermolecular forces of attraction in order to contain the molecules together. However, these forces are not strong enough to hold them into definite positions. Liquids possess indefinite shape and fluidity.
Question 5: Why is there no increase in the volume of water, when sugar is dissolved in it?
Answer:
Liquids don’t display an increase in volume even after the addition of sugar. This is because the sugar particles occupy the spaces in between the spaces contained within the liquid molecules.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...