Preparation of Amines
Last Updated :
11 Feb, 2022
Amines are organic compounds formed by substituting an alkyl or aryl group for one or more hydrogen atoms in an ammonia (NH3) molecule. They can be found in nature in proteins, vitamins, alkaloids, and hormones. Synthetic materials include polymers, dyes, and pharmaceuticals. Blood pressure is raised using two biologically active compounds, adrenaline, and ephedrine, both of which contain a secondary amino group. Benadryl, a well-known antihistaminic drug, also contains a tertiary amino group. Surfactants made from quaternary ammonium salts are used. Diazonium salts are used as intermediates in the production of a wide range of aromatic compounds, including dyes.
Amines are ammonia derivatives formed by substituting alkyl and/or aryl groups for one, two, or all three hydrogen atoms. Amines include the following:
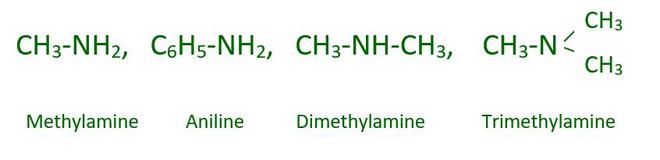
Preparation of Amines
There are different methods to prepare amines:
- From reduction of nitro compound
By passing hydrogen gas through a finely divided nickel, palladium, or platinum, as well as by reduction with metals in an acidic medium, nitro compounds are reduced to amines. Nitroalkanes can also be reduced to the corresponding alkanamines in the same way.
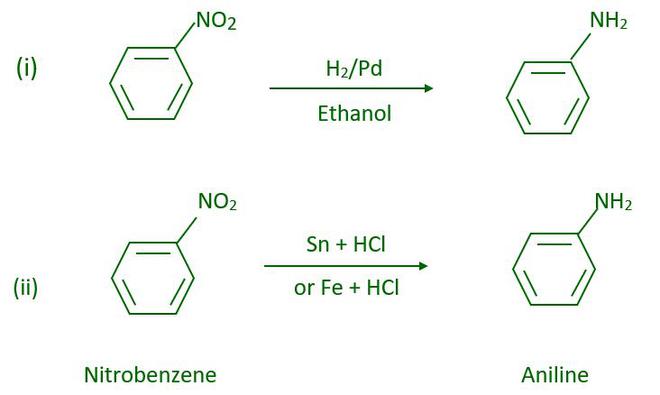
Reduction with iron scrap and hydrochloric acid is preferred because the FeCl2 formed during the reaction is hydrolyzed, releasing hydrochloric acid. As a result, only a trace of hydrochloric acid is required to initiate the reaction.
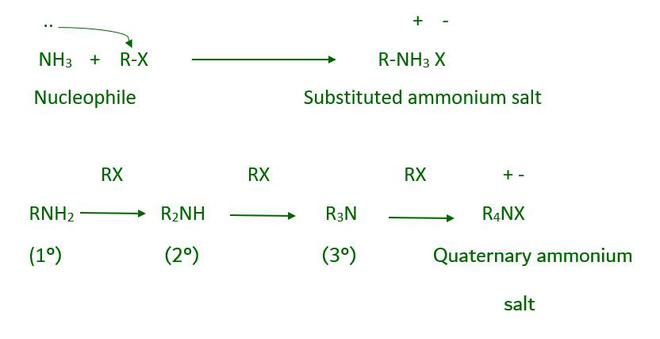
- From ammonolysis Alkyl halide
The bond between carbon-halogen and alkyl or benzyl halides can be easily broken by a nucleophilic reaction. As a result, when an alkyl or benzyl halide reacts with an ethanolic solution of ammonia, a nucleophilic substitution reaction occurs in which the halogen atom is replaced by an amino (–NH2 ) group. Ammonolysis is the process by which an ammonia molecule cleaves the C–X bond. The reaction takes place in a sealed tube at 373 K. The resulting primary amine is a nucleophile and can react with an alkyl halide to form secondary and tertiary amines, and finally quaternary ammonium salt.
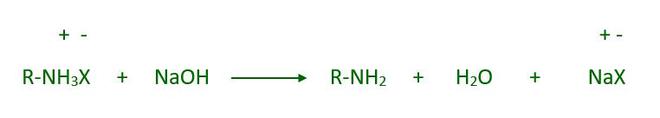
By treating the ammonium salt with a strong base, the free amine can be obtained:
Ammonolysis of alkyl halide has one disadvantage during the reaction it forms a mixture of primary, secondary, and tertiary amines and quaternary ammonium so to get Primary amine, as a major product we pass a large excess of ammonia.
- From reduction of Nitriles
Primary amines are formed when nitrites are reduced with lithium aluminum hydride (LiAlH4) or catalytically hydrogenated. This reaction is used to prepare amines with one more carbon atom than the starting amine, which is known as amine series ascent.
H2/Ni
R-CN ————————-> R-CH2-NH2
Na(Hg) /C2H5OH
From reduction of Amide
When amides are reduced with lithium aluminum hydride, amines are formed.
(i) LiAlH4
R-CO-NH2 —————————–> R-CH2-NH2
(ii) H2O
- From Gabriel phthalimide Synthesis
Gabriel synthesis is a method for producing primary amines. When phthalimide is treated with ethanolic potassium hydroxide, it forms a potassium salt of phthalimide, which when heated with an alkyl halide and then alkaline hydrolyzed yields the corresponding primary amine. Gabriel’s phthalimide synthesis method does not produce aromatic primary amines because aryl halides do not undergo nucleophilic substitution reaction with the anion formed by phthalimide.

- From Hoffmann bromamide degradation reaction
Hoffmann developed a method for producing primary amines by treating an amide with bromine in an aqueous or ethanolic sodium hydroxide solution. An alkyl or aryl group migrates from the amide’s carbonyl carbon to the nitrogen atom during this degradation reaction. The amine thus formed has one fewer carbon atom than the amide.
R-CO-NH2 + Br2 + 4NaOH —————————–> R-NH2 + Na2CO3 + 2NaBr + 2H2O
(Amide) (Sodium Hydroxide) (Amine)
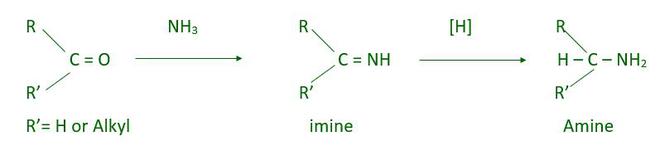
- Reductive animation of aldehydes and ketones
Reductive animation of aldehydes and ketones produces 1°, 2°, and 3° amine. This reaction takes place in 2 step first is nucleophilic addition which gives imine and the second step is the reduction of imine which produce amine.
Curtius reaction is also called Curtius rearrangement. In this reaction decomposition of acyl azide takes place which forms isocyanate. Isocyanate is unstable which on hydrolysis gives amine.
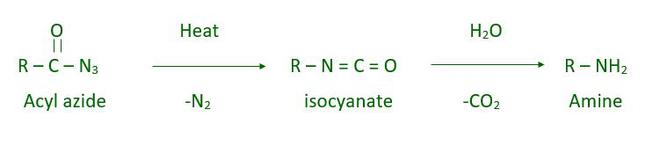
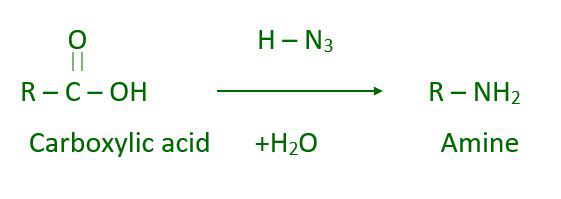
In Schmidt, reaction amines can be prepared with azide and a carboxylic acid.
Sample Problems
Question 1: What are primary, secondary, and tertiary amines? give examples.
Answer:
One hydrogen atom of NH3 is replaced with an alkyl or aryl group in primary or 1° amine. Two hydrogen atoms of NH3 are replaced with an alky or aryl group in secondary or 2° amine. Three hydrogen atoms of NH3 are replaced with an alkyl or aryl group in tertiary amine.
Example: CH3-NH2, CH3-NH-CH3, CH3-N-(CH3)2
Methyl Amine (1°) Dimethyl Amine (2°) Trimethyl Amine (3°)
Question 2: Write the IUPAC name of,
- CH3–CH2–NH2
- CH3–CH2–CH2–NH2
- C6H5-NH2.
Answer:
The IUPAC name are-
- CH3–CH2–NH2 – Ethanamine
- CH3–CH2–CH2–NH2 – Propan-1-amine
- C6H5-NH2 – Benzenamine.
Question 3: What happens when nitrobenzene is reduced? Write the chemical equation of the reaction.
Answer:
Reduction of nitro benzene gives aniline as final product. Chemical equation-

Question 4: What is the method to prepare ethylamine in the lab?
Answer:
Hofmann’s bromide reaction is used In the laboratory, we can make ethylamine using Hofmann’s bromide reaction. Bromine and potassium hydroxide solution are used to heat the propionamide.
Question 5: Which is more soluble in water primary amine or tertiary amines give a reason?
Answer:
Tertiary amines are more soluble in water because primary amines can form hydrogen bonds with water but tertiary amines cannot, primary amines are less soluble.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...