Phosphine – Structure, Preparation, Properties, Uses
Last Updated :
29 Jan, 2022
The final electron of a P block element enters one of the three p-orbitals of the shell in which it is found. There are six groups of p-block elements since a p-subshell has three degenerate p-orbitals, each of which may hold two electrons.
Because of their tendency to lose an electron, P block elements are lustrous and typically strong conductors of electricity and heat. In a P-block element like gallium, you may find some astonishing characteristics of elements. It’s a metal that melts in your palm. Because silicon is a key component of glass, it is also one of the most significant metalloids in the p-block group.
Phosphine
Phosphine is a phosphorus hydride with the formula PH3. Phosphine is a hazardous gas that is inflammable. It is commonly made in enterprises and labs due to its important industrial applications.
Phosphine may be found in human blood, urine, and saliva in nature.
Structure and Formula of Phosphine
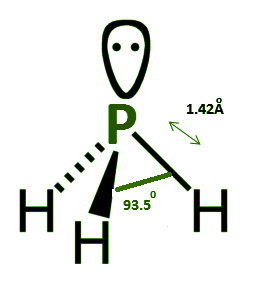
Phosphine has a trigonal pyramidal structure. The lone pair of electrons present on phosphorus causes the molecule’s symmetry to alter. sp3 hybridization occurs in phosphorus. Three bound pairs and one lone pair of electrons are present. The angle of the H–P–H bond is 93.5o, while the length of the P–H bond is 1.42 pm.
Preparation of Phosphine
- Hydrolysis of metal phosphides with calcium phosphide- Metal phosphides react with water or HCl to produce phosphine.
Ca3P2+6H2O→2PH3+3Ca(OH)2
Ca3P2+6HCl→2PH3+3CaCl2
- From phosphorus acid- Pure phosphine is formed via the breakdown of H3PO3.
4H3PO3→3H3PO4+PH3
- Preparation from Phosphorus- Heating white phosphorus in a concentrated NaOH solution in an inert CO2 environment produces PH3.
P4+3NaOH+3H2OPH3+3NaH2PO2
Purification of Phosphine
Pure phosphine is a non-flammable substance. Due to the presence of P2H4 or P4 vapours, it becomes combustible. Phosphine is refined by absorbing it in HI and converting it to PH4l. When you combine this with potassium hydroxide, you get phosphine.
PH4I+KOH→PH3+KI+H2O
Physical Properties of Phosphine
- It’s a colourless gas that’s extremely dangerous.
- As previously stated, phosphorus in its pure form is non-flammable. It is, nevertheless, combustible due to the presence of P4 vapour. When phosphorus comes into touch with oxidising substances like HNO3, chlorine, or bromine vapours, it can catch fire. It can catch fire even if just a little amount or traces of these oxidising chemicals are present.
- It has a distinct rotten fish odour to it.
- It’s just slightly soluble in water.
- It has a boiling point of –87.7∘ Celsius.
Chemical Properties of Phosphine
- Phosphine, like ammonia, is a Lewis base. When it reacts with HI, HBr, and HCl, it produces phosphonium compounds.
PH3+Hl→PH4l
PH3+HBr→PH4Br
PH3+HCl→PH4Cl
- Combustibility- When phosphorus is burned, it produces phosphorus pentoxide and water.
2PH3+4O2→P2O5+3H2O
PH3+4Cl2→PCl5+3HCl
- Under normal circumstances, it will not ignite. It explodes with flame when heated, releasing phosphoric acid.
- When exposed to oxidising substances such as HNO3, Cl2 and Br2, it explodes dramatically.
- PH3 in water decomposes in the presence of light to produce red P and H2O.
PH3(H2O)→P(red)+H2O
- Copper phosphide and mercuric phosphide are generated when phosphine is absorbed in copper sulphate or mercuric chloride.
3CuSO4+2PH3→Cu3P2+3H2SO4
3HgCl2+2PH3→Hg3P2+6HCl
Uses of Phosphine
- Phosphine is a chemical that is used to make metallic phosphides.
- Holme’s signal and Smokescreens contain phosphorus.
Holme’s signal:
The fact that phosphine spontaneously combusts makes it a likely candidate for use in Holme’s signal. When ships require assistance, calcium carbide and calcium phosphide-filled containers are pierced and tossed into the water. Acetylene and phosphine gases are produced as a result of the interaction with water. These gases emit a signal when they burn in the air.
Smoke screen:
When calcium phosphide is soaked in water, it creates a considerable amount of phosphine, resulting in a smoke screen. During the conflict, this was employed to hide the troops from the enemy.
In rodent and insect control formulations, phosphine fumigants are utilised.
Sample Questions
Question 1: What are the properties of phosphine?
Answer
- Phosphine is a colourless, very toxic gas.
- It has a distinctive rotten-fish odour.
- It has a low water solubility.
Question 2: What are the uses of phosphine?
Answer
Phosphine is employed in the smoke screen, Holme’s signal, and insecticides and rodenticides as a fumigant.
Question 3: What is the structure of phosphine?
Answer
With a lone pair of electrons on P, the structure is trigonal bipyramidal. Three bound pairs and one lone pair of electrons are present. The angle of the connection is 93.5 degrees. The length of the P–H bond is 1.42 Armstrong.
Question 4: How is phosphine gas prepared in the laboratory?
Answer
White phosphorus is burned with a strong NaOH solution in a CO2 inert environment to produce phosphine in the laboratory.
P4+3NaOH+3H2O→PH3+3NaH2PO2
Question 5: How to purify phosphine?
Answer
Phosphine is refined by absorbing impurities in HI, yielding PH4l, which is then treated with potassium hydroxide to get phosphine.
PH4l+KOH→KI+H2O+PH3
Question 6: What is Phosphine?
Answer
Phosphine has the formula PH3 and is a phosphorus hydride. Phosphine is a volatile and dangerous gas. Because of its vital industrial applications, it is routinely manufactured in businesses and laboratories.
In nature, phosphine may be found in human blood, urine, and saliva.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...