NCERT Solutions for Class 11 Biology Chapter 9 – Biomolecules
Last Updated :
22 May, 2023
NCERT Solutions for class 11 Biology Chapter 9 Biomolecules: The chapter on Biomolecules is important for students approaching the board exams. This article introduces NCERT solutions designed to help students explain the concepts of further learning and how to write to get good grades on exams. The solutions are presented in very simple language for ease of understanding.
NCERT CBSE Chapter 9 of Class 11 Biology Biomolecules explains the characteristics of biomolecules like carbohydrates, proteins and nucleic acids and hormones. This chapter further explains the concept of Biomolecules in detail. Revise the basic concepts of Biomolecules for quick revision and class notes.
Q1: What are macromolecules? Give examples.
Answer:
Large complex molecules, such as nucleic acids, proteins, carbohydrates, and lipids, with relatively large molecular weight. Examples:
- Carbohydrates: Glucose, sucrose, starch, cellulose, chitin.
- Lipids: Fats, phospholipids, oils, steroids.
- Proteins: Keratins, hormones, enzymes.
- Nucleic acid: DNA, RNA.
Q2: What is meant by the tertiary structure of proteins?
Answer:
The long protein chain folded upon itself like a hollow woollen ball, which gives us a 3-dimensional view of a protein is known as a tertiary structure of the protein. Tertiary structure is absolutely necessary for the many biological activities of proteins.
Q3: Find and write down structures of 10 interesting small molecular weight biomolecules. Find if there is any industry which manufactures the compounds by isolation. Find out who are the buyers.
Answer:
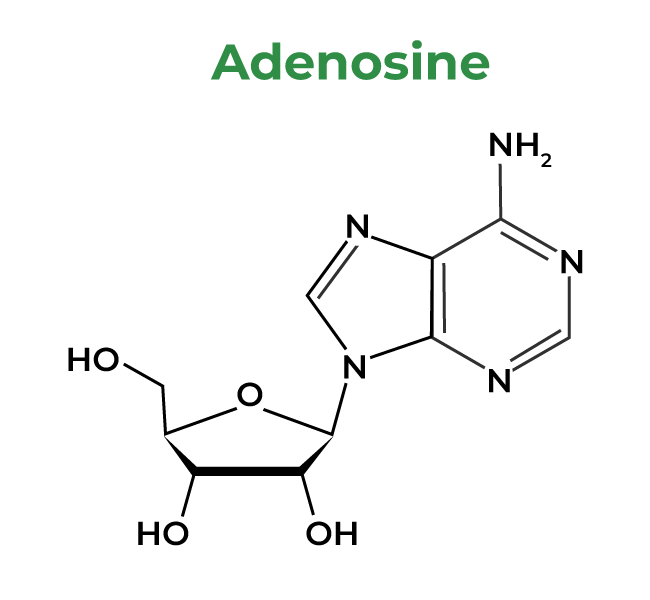
1: Adenosine
2: Lactose

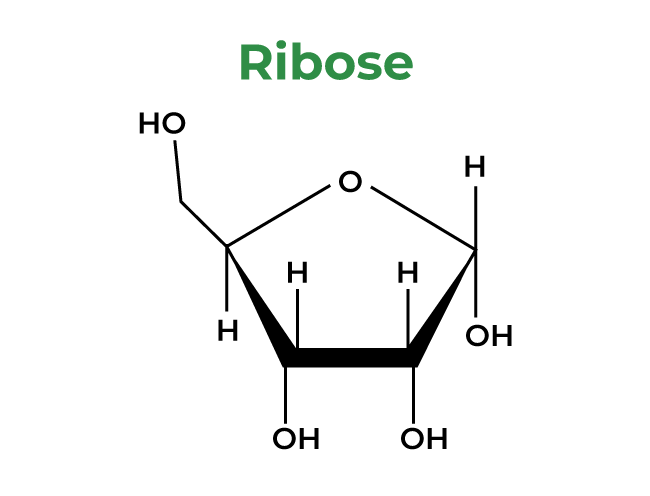
3. Ribose
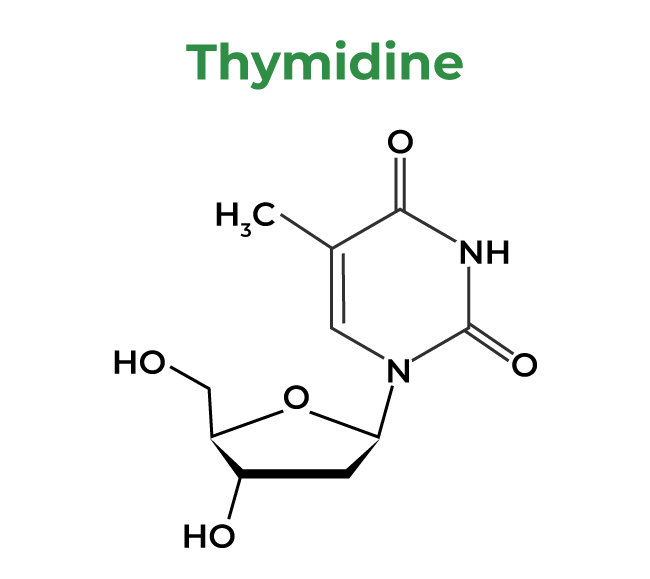
4. Thymidine
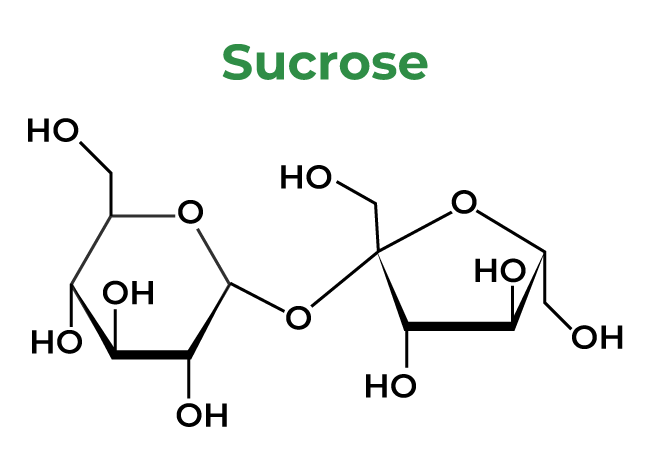
5: Sucrose
6: Maltose
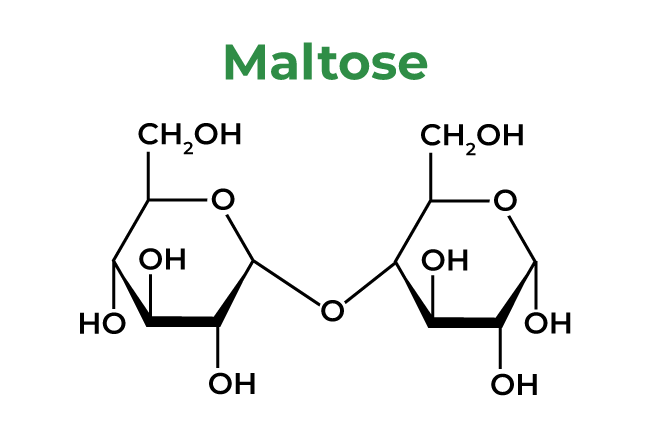
7: DNA

8: RNA
9: Glycerol

10: Insulin
|
Compound
|
Manufacturer
|
Buyer
|
|
Starch
|
Premier starch products pvt. ltd.
|
Research institutes and laundries
|
|
Lipid glucose
|
Imperial liquid glucose
|
Used in making flavoured drinks and research
|
|
Enzymes like amylase, protease and cellulase
|
Planet Biotech India
|
Used in research
|
Q4: Find out and make a list of proteins used as therapeutic agents. Find other applications of proteins (e.g., Cosmetics etc.)
Answer:
Proteins used as therapeutic agents are as follows:
- Insulin – It helps in maintaining blood glucose levels in the body.
- Antigen (antibody) – It helps in blood transfusion.
- Thrombin and fibrinogen – They help in blood clotting.
- Renin – It helps in osmoregulation.
Proteins are also commonly used in the manufacture of toxins and as biological buffers.
Q5: Explain the composition of triglyceride.
Answer:
A triglyceride is composed of glycerol and three fatty acids. Triglycerides are the predominant component of most food fats and oils. The minor components include mono- and diglycerides, free fatty acids, phosphatides, sterols, fatty alcohols, fat-soluble vitamins, and other substances.
Structure of Triglyceride:

Q6: Can you attempt building models of Biomolecules using commercially available Atomic Models (Ball and Stick Models)?
Answer:
Yes, the ball and stick model can be used to represent biomolecules. Sticks depict the bonds that hold the molecule together, while balls represent the atoms. A model of D-glucose is shown below, with hydrogen atoms represented by green balls, oxygen atoms represented by pink balls, and carbon atoms represented by grey balls.

Q7: Draw the structure of the amino acid, alanine.
Answer:

Q8: What are gums made of? Is Fevicol different?
Answer:
Guns are made from two or more different types of monosaccharides. So, they are hetero-polysaccharides. Whereas, fevicol is polyvinyl alcohol (PVA) glue and is not a polysaccharide.
Q9. Find out a qualitative test for Proteins, Fats and Oils, and Amino acids and test any fruit juice, saliva, sweat and urine for them.
Answer:
(a) Protein – Biuret’s test
When Biuret’s reagent is added to the protein, the colour of the reagent changes from light blue to purple.
(b) Fats and oils – Emulsion test
Add ethanol to a very small amount of the test substance. Shake in order to dissolve. Filter or dilute to obtain fairly clear liquid. Take another tube containing water and pour the ethanolic solution into the top. A white (milk like) emulsion indicates the presence of fats or oils.
(c) Amino acid – Ninhydrin test
When Ninhydrin reagent is added to the solution, the colourless solution changes to pink, blue, or purple, depending on the amino acid.
1) Fruit juice
• Biuret’s test – Fruit juice + Biuret’s reagent – Colour changes from light blue to purple. Protein is present.
• Grease test – To a brown paper, add a few drops of fruit juice – No translucent spot. Fats and oils are absent or are in negligible amounts
• Ninhydrin test – Fruit juice + Ninhydrin reagent + boil for 5 minutes – Colourless solution changes to pink, blue, or purple colour. Amino acids are present.
2) Saliva
• Biuret’s test – Saliva + Biuret’s reagent – Colour changes from light blue to purple. Proteins are present.
• Grease test – On a brown paper, add a drop of saliva – No translucent spot. Fats/oils are absent.
• Ninhydrin test – Saliva + Ninhydrin reagent + boil for 5 minutes – Colourless solution changes to pink, blue, or purple colour. Amino acids are present.
3) Sweat
• Biuret’s test – Sweat + Biuret’s reagent- No colour change. Proteins are absent.
• Solubility test – Sweat + Water – Oily appearance – Fats/oil may be present.
• Ninhydrin test – Sweat + Ninhydrin reagent + boil for 5 minutes – No colour change, the solution remains colourless. Amino acids are absent.
4) Urine
• Biuret’s test – Few drops of urine + Biuret’s reagent – Colour changes from light blue to purple- Proteins are present.
• Solubility test – Few drops of urine + Water – Little bit of oily appearance. Fats may or may not be present.
• Ninhydrin test – Few drops of urine + Ninhydrin reagent + boil for 5 minutes. Colourless solution changes to pink, blue, or purple colour depending on the type of amino acid- Amino acids are present.
Q10: Find out how much cellulose is made by all the plants in the biosphere and compare it with how much paper is manufactured by man and hence what is the consumption of plant material by man annually. What a loss of vegetation!
Answer:
Earth’s plants produce at least 100 billion tonnes of cellulose each year and about 400 million tonnes of paper is manufactured by man. So, the loss of vegetation is very high in the case of the consumption of plant material by man annually.
Q11: Describe the important properties of enzymes.
Answer:
Four important properties of enzymes are:
- Catalytic Property: Enzymes have extraordinary catalytic power. They are active in very small quantities. A small amount of enzyme is enough to convert a large quantity of substrate and the enzymes remain unchanged after the reaction.
- Specificity: Enzymes are very specific in their action. Particular enzymes act on particular substrates only. Enzymes are also specific to a particular type of reaction. In some rare cases, the specificity may not be too strong.
- Reversibility: Most of the enzyme-catalysed reactions are reversible. The reversibility of the reaction depends upon the requirements of the cell. In some cases, there are separate enzymes for forward and reverse reactions. Some enzyme-catalysed reactions are not reversible.
- Sensitiveness to heat and temperature and pH: Enzymes are susceptible to heat and temperature. They are thermolabile. Enzymes will be inactive at very low temperatures; this is the reason for preserving food and vegetables in the refrigerator. The enzymatic activity increases with the increase in temperature up to a certain level. At higher temperatures (60-70 degree Celsius), the enzyme is destroyed or denatured. The optimum pH of most endo-enzyme is pH 7.0 (neutral pH). However, digestive enzymes can function at different pH.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...