Molybdic Acid Formula
Last Updated :
20 Dec, 2023
Molybdenum is a chemical element present in the periodic table. It is represented with the symbol Mo. The atomic number of Molybdenum is 42. It is which is located in period 5 and is part of group 6. This element was first discovered in the year 1778 by Carl Wilhelm Scheele. and it was isolated successfully for the first time in 1781 by Peter Jacob Hjelm. It is not present in its free state in nature and is only found in the forms of ores. When it is in its free state it is a silver metal with a grey cover.
What is Molybdic acid?
Molybdic acid is the hydrated form of molybdenum trioxide and some other related species. Molybdic acid can also be called molybdenum hydroxide oxide or molybdic(VI) acid, or dihydrogentetraoxomolybdate. It is mostly found in two forms monohydrate and dihydrates based on the number of water molecules present in this chemical. The chemical formula of the monohydrate is MoO3·H2O and dihydrate is MoO3·2H2O. This substance id is diamagnetic in nature. It appears as a yellow-coloured solid.
Structure of Molybdic acid
Molybdic acid is the hydrated form of molybdenum trioxide and some other related species. Molybdic acid can also be called molybdenum hydroxide oxide or molybdic (VI) acid. The chemical formula of the monohydrate is MoO3·H2O and dihydrate is MoO3·2H2O. Molybdic acid is a molybdenum oxoacid. It is the conjugate acid of a hydrogenmolybdate. The IUPAC name of molybdic acid is dihydrogentetraoxomolybdate. The hydrogen donor and acceptor count in this molecule are 2 and 4 respectively.

Molybdic acid structure
Preparation of Molybdic acid
- If the pH of the molybdate ion is dropped below 1 it converts into molybdic acid.
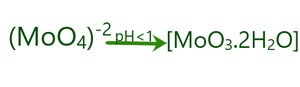
Preparation of Molybdic acid
- It can be prepared by adding 2 grams of ammonium molybdate tetrahydrate, (NH4)6Mo7O24·4H2O, in 28 mL of distilled water. Then remove the nitrogen.
Physical Properties of Molybdic Acid
- It mostly appears as a solid or in powder form.
- It is of pale yellow or white color.
- The relative density of molybdic acid is 3.124.
- It is slightly soluble in water.
- It is highly soluble in alkalic solutions.
- The melting point of Molybdic acid is 300o C.
- The solubility is 70 grams per liter.
Chemical Properties of Molybdic Acid
- The molecular mass of molybdic acid is 161 amu.
- The molecular formula of molybdic acid is MoO3·H2O.
- When pyrocatechol reacts with molybdic acid it gives rise to MoO2 ion and 1,2-Cyclohexanedione.

Molybdic acid preparation
Uses of Molybdic Acid
- Molybdic acid is used as a catalysts in chemical reactions.
- Molybdic acid is used to produce Froehde reagent.
- Molybdic acid is used as a precursor in the production process of molybdenum(IV) sulfide and ammonium molybdate tetrahydrate.
- Mixture of Molybdic acid and sulfuric acid is used to detect the presence of alkaloids.
- It is used to make ammonium molybdate.
FAQs on Molybdic Acid Formula
Question 1: Write a short note on Molybdenum.
Answer:
Molybdenum is a chemical element. It is represented with the symbol Mo and has atomic number 42. It is which is located in period 5 and group 6. This element was first discovered in the year 1778 by Carl Wilhelm Scheele. and it was isolated successfully in for the first time in 1781 by Peter Jacob Hjelm.
Question 2: Briefly discuss Molybdic acid.
Answer:
Molybdic acid is the hydrated form of molybdenum trioxide and some other related species. Molybdic acid can also be called molybdenum hydroxide oxide or molybdic(VI) acid, or dihydrogentetraoxomolybdate. It is mostly found in two forms monohydrate and dihydrates. This substance id is diamagnetic yellow colored solid.
Question 3: What is ammonium molybdate And what are its uses?
Answer:
Ammonium Molybdate is a derivative of the molybdic acid. It is used as the main raw material in the production of molybdenum wire, ceramic glaze and many other molybdenum compounds. Ammonium Molybdate is used as agricultural fertilizer, pigments, fabric fire retardant agent and pigments. It can be used in ceramics and paints. Intake of Ammonium Molybdate may be harmful for the digestive tract.
Question 4: Is molybdenum toxic to human beings?
Answer:
When the intake is in less quantity molybdenum is not considered toxic but rather healthy. Small quantities of molybdenum is essential for the wellbeing of humans. Appropriate Quantity of molybdenum is helpful in reducing the blood pressure and also is good for the liver.
Question 5: Briefly Explain the structure of molybdic acid.
Answer:
Molybdic acid is the hydrated form of molybdenum trioxide and some other related species. Molybdic acid is a molybdenum oxoacid. It is conjugate acid of a hydrogenmolybdate. The IUPAC name of molybdic acid is dihydrogentetraoxomolybdate. The hydrogen donor and acceptor count in this molecule is 2 and 4 respectively.
Question 6: What are the main applications of molybdic acid?
Answer:
Molybdic acid is used as a catalysts in chemical reaction. It is used to make ammonium molybdate. Molybdic acid is used as precursor in the production process of molybdenum(IV) sulfide and ammonium molybdate tetrahydrate. Molybdic acid is used to produce Froehde reagent. Mixture of Molybdic acid and sulfuric acid is used to detect the presence of alkaloids.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...