Intrinsic Semiconductors and Extrinsic Semiconductors
Last Updated :
26 Sep, 2021
A semiconductor substance has an electrical property that sits between an insulator and a conductor. Si and Ge are the greatest examples of semiconductors. There are two types of semiconductors: intrinsic semiconductors and extrinsic semiconductors (p-type and n-type). The intrinsic kind of semiconductor is pure, but the extensive type contains impurities to make it conductive. At ambient temperature, intrinsic conductivity will be nil, but extrinsic conductivity will be minimal. With doping and energy band diagrams, this article provides an overview of intrinsic and extrinsic semiconductors.
Intrinsic Semiconductor
The definition of an intrinsic semiconductor is a semiconductor that is exceedingly pure. According to the energy band theory, the conductivity of this semiconductor will be zero at ambient temperature. Si and Ge are two examples of intrinsic semiconductors.
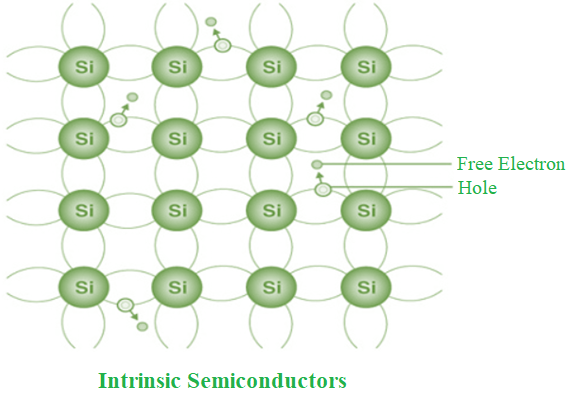
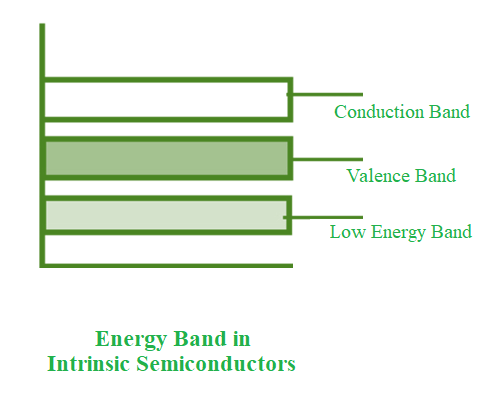
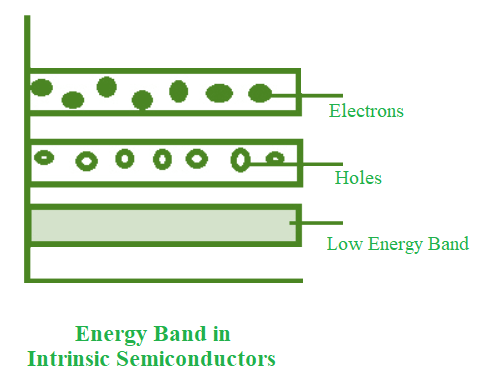
- The conduction band is empty in the below energy band diagram, but the valence band is completely filled. Some heat energy can be provided to it once the temperature has been raised. As a result of exiting the valence band, electrons from the valence band are provided to the conduction band.
- The flow of electrons will be random as they go from the valence to the conduction band. The crystal’s holes can also flow freely in any direction.
- As a result, the TCR of this semiconductor will be negative (temperature coefficient of resistance). The TCR indicates that when the temperature rises, the material’s resistance decreases and its conductivity rises.
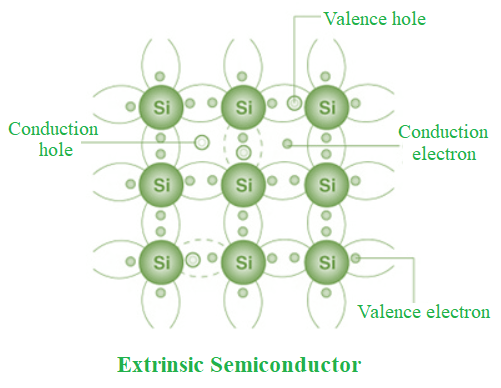
Extrinsic Semiconductor
Extrinsic semiconductors are semiconductors that have had an impurity introduced to them at a regulated rate to make them conductive.
- While insulating materials may be doped to make them into semiconductors, intrinsic semiconductors can also be doped to make an extrinsic semiconductor.
- Extrinsic semiconductors are divided into two categories as a result of doping: atoms with an additional electron (n-type for negative, from group V) and atoms with one fewer electron (p-type for positive, from group III).
- Doping is the purposeful introduction of impurities into a very pure, or intrinsic, semiconductor in order to change its electrical characteristics. The kind of semiconductor determines the impurities. Extrinsic semiconductors are those that are light to moderately doped.
What is Doping?
Doping is the process of introducing an impurity into a semiconductor. During the production of extrinsic semiconductors, the amount and kind of impurity to be introduced to the material must be carefully monitored. In most cases, one impurity atom is introduced to every 108 semiconductor atoms.
Impurity is used to enhance the number of free electrons or holes in a semiconductor crystal, making it more conductive. A significant number of free electrons will exist if a pentavalent impurity with five valence electrons is introduced to a pure semiconductor. A significant number of holes will exist in the semiconductor if a trivalent impurity with three valence electrons is introduced. Extrinsic semiconductors are divided into two categories based on the type of impurity added: N-type and P-type semiconductors.
n-Type Semiconductors
N-type semiconductors are extrinsic semiconductors in which dopant atoms can provide additional conduction electrons to the host material (e.g. phosphorus in silicon).
An overabundance of negative (n-type) electron charge carriers results as a result of this. Doping atoms often contain one extra valence electron than the host atoms. Atomic replacement in group-IV solids by group-V elements is the most typical case. When the host includes many types of atoms, the issue becomes more complicated. Silicon, for example, can act as a donor when it replaces gallium or as an acceptor when it replaces arsenic in III-V semiconductors like gallium arsenide. Some donors have fewer valence electrons than the host, such as alkali metals, which are donors in most solids.
p-Type Semiconductors
To enhance the number of free charge carriers, a p-type (p for “positive”) semiconductor is formed by adding a certain type of atom to the semiconductor.
The doping substance removes (accepts) weakly bonded outer electrons from semiconductor atoms when it is introduced. The vacancy left behind by the electron is known as a hole, and this sort of doping agent is also known as an acceptor substance. The goal of p-type doping is to produce a large number of holes.
In the instance of silicon, the crystal lattice is swapped by a trivalent atom. As a result, one of the four covalent bonds that typically make up the silicon lattice is lacking an electron. As a result, the dopant atom can accept an electron from the covalent link of a nearby atom to complete the fourth bond. Acceptors are the name given to these dopants because of this.
When a dopant atom takes an electron, it causes the nearby atom to lose half of its link, resulting in the creation of a hole. Each hole is connected to a neighbouring negatively charged dopant ion, resulting in an electrically neutral semiconductor. Once each hole has strayed away into the lattice, one proton in the atom at the hole’s position will be “exposed,” meaning it will no longer be cancelled by an electron. This atom will contain three electrons and one hole in its nucleus, which will have four protons.
As a result, a hole behaves like a positive charge. When a large enough number of acceptor atoms are supplied, thermally excited electrons are substantially outnumbered by holes. In p-type materials, holes are the majority carriers, whereas electrons are the minority carriers.
Difference between Intrinsic and Extrinsic Semiconductors
The following are some of the key distinctions between extrinsic and intrinsic semiconductors:
- Intrinsic semiconductors exist in their purest form at all times while Extrinsic semiconductors are created by doping impurities in pure semiconductors.
- At room temperature, Intrinsic semiconductors have poor electrical conductivity while Extrinsic semiconductors have a high electrical conductivity compared to other materials.
- The number of electrons equals the number of holes in Intrinsic semiconductors while numbers are unequal in Extrinsic semiconductors.
- Intrinsic semiconductors are solely reliant on temperature while Extrinsic semiconductors are affected by temperature and the number of contaminants present.
- Intrinsic semiconductors are not further classified while N-type and p-type semiconductors are two types of semiconductors in Extrinsic semiconductors.
- Silicon and germanium are two examples of Intrinsic semiconductors while Si and Ge doped with Al, In, P, As, and other elements are examples of Extrinsic semiconductors.
Sample Questions
Question 1: What is an n-type semiconductor?
Answer:
When a tetravalent element such as Silicon or Germanium is doped with a pentavalent element such as Arsenic (As) or Antimony, the result is an n-type semiconductor (Sb). Thus in the crystal lattice, one atom of the pentavalent element takes the place of an atom of the four valent elements.
All five pentavalent atom electrons establish strong connections with their tetravalent neighbours, and the fifth electron creates a weak bond with its parent element after the doping process is complete. A relatively little amount of energy is needed to ionise the fifth electron. Although it is in the tetravalent element’s crystal structure, the fifth electron is also free to roam about even at room temperature.
Question 2: What is a P-type semiconductor?
Answer:
When a tetravalent element such as silicon or germanium is doped with a three-valent element such as aluminium (Al), indium (In), etc., the result is a P-type semiconductor. After doping, three of the tetravalent element’s four electrons establish a covalent connection with the trivalent element’s three electrons. There is a deficit of one electron, and as a result, the fourth electron has no electron with which to bind.
As a result, a void or hole is produced, and it becomes necessary to fill it. As a result, an electron in the outer orbit of a nearby atom has a chance to leap and fill the void. In this way, one electron is removed from the system, leaving a void or a hole in its stead. Conduction can then occur through the hole.
Question 3: What is the result of doping germanium metal with a little quantity of indium?
Answer:
P-type semiconductors are made from germanium impurities that include indium. Impurities of a trivalent nature can be added to germanium to generate the P-type material. They are called acceptor impurities because they are trivalent.
Question 4: What is the name of a pure semiconductor crystal in which current flows owing to the breaking of crystal bonds?
Answer:
Intrinsically pure semiconductors are referred to be that. The conduction band has an identical amount of electrons as it has holes and vice versa. In addition to being termed intrinsic semiconductors, undoped semiconductors and i-type semiconductors are other names for intrinsic semiconductors.
Question 5: In which of the following situations do holes make up the majority of the current?
Answer:
The bulk of charge carriers in p-type extrinsic semiconductors are holes, which are amorphous semiconductors. They are called acceptor impurities because they are trivalent. The minority charge carriers in p-type semiconductors are electrons.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...