In chemistry, a hydrogen bond is an electrostatic force of attraction between a hydrogen atom and another electronegative atom. It is a special type of dipole-dipole force. Hydrogen bonding is the phenomenon of the formation of Hydrogen Bonds.
H Bonds are stronger than any dipole-dipole bonds but weaker than covalent bonds. Hydrogen bonds can form between atoms within a molecule or between two different molecules.
Hydrogen bonds can form when:
- A hydrogen atom is covalently bonded to a more electronegative atom
- The other atom has a lone pair of electrons
Examples of hydrogen bonds Water, Ammonia, and Hydrogen Fluoride.
What is Hydrogen Bond?
A hydrogen bond is a type of electrostatic force of attraction between a hydrogen atom and another electronegative atom. It is a type of intermolecular force. The hydrogen atom is covalently bonded to a more electronegative “donor” atom or group, and the other atom has a lone pair of electrons. The hydrogen atom is called the H-bond donor, and the other atom is called the H-bond acceptor.
Hydrogen bonds are weak, intermolecular bonds that hold together soft matter, water, network liquids, and many ferroelectric crystals. For example, water is a liquid over a wider range of temperatures than expected for its size because of its extensive hydrogen bonding. Water is also a good solvent for ionic compounds and many others because it readily forms hydrogen bonds with the solute.
Formation of Hydrogen Bonds
Hydrogen bonds form when a hydrogen atom that is covalently bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) interacts with another electronegative atom nearby. The formation of hydrogen bonds is illustrated as follows:
- Polar Covalent Bond: Initially, there is a polar covalent bond between a hydrogen atom (H) and an electronegative atom (let’s say oxygen, O). In a water molecule (H2O), for example, each hydrogen atom forms a polar covalent bond with the oxygen atom.
- Electronegativity Difference: Oxygen is more electronegative than hydrogen. As a result, the shared electrons in the covalent bond spend more time around the oxygen nucleus, creating a partial negative charge (δ−) on the oxygen atom and a partial positive charge (δ+) on the hydrogen atom.
- Attraction between Opposite Charges: The partial positive charge on the hydrogen atom in one molecule is attracted to the partial negative charge on the electronegative atom of another molecule. For example, the partial positive charge on the hydrogen atom of one water molecule is attracted to the partial negative charge on the oxygen atom of another water molecule.
- Formation of Hydrogen Bond: This electrostatic attraction between the partially positive hydrogen atom and the partially negative atom of another molecule is known as a hydrogen bond. Although individually weaker than covalent or ionic bonds, hydrogen bonds collectively play crucial roles in various biological and chemical processes, such as the structure of DNA, the properties of water, and protein folding.
In short, hydrogen bonds form due to the attraction between the partial positive charge on a hydrogen atom and the partial negative charge on an electronegative atom of another molecule, leading to the formation of a weak electrostatic bond between the molecules.
What is Hydrogen Bonding?
Hydrogen Bonding is simply the formation of Hydrogen bonds. Hydrogen bonding is a type of chemical bonding that possess an electrostatic force of attraction between a hydrogen atom and an atom containing a lone pair of electrons in a chemical substance. The hydrogen must be attached to an electronegative atom for a hydrogen bond to form. It should be noted that the hydrogen bond is not a covalent bond however, the atoms present in it are covalent.
Hydrogen bonding is the formation of hydrogen bonds. Hydrogen bonds are the type of attractive intermolecular forces caused by the dipole-dipole interaction between a hydrogen atom bonded to a strongly electronegative atom of the same or another nearby electronegative atom.
Hydrogen is covalently bound to the more electronegative oxygen atom in water molecules (H2O). As a result of dipole-dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another H2O molecule, hydrogen bonding occurs in water molecules.
The bond pair of electrons in the O-H bond is quite close to the oxygen nucleus in this case (due to the large difference in the electronegativities of oxygen and hydrogen). As a result, the hydrogen atom generates a partial positive charge (+) whereas the oxygen atom develops a partial negative charge (-).
Important Conditions for Hydrogen Bonding
Hydrogen Bonding is possible in cases when the molecule contains a highly electronegative atom linked to the hydrogen atom. The hydrogen bonding in water is a vigorous bond between the nearest water molecule containing one Hydrogen atom between two oxygen atoms. Hydrogen bonding is major of two types of intramolecular and intermolecular hydrogen bonding, on the basis of the atoms involved in it. Hydrogen bonding is maximum in solid-state compounds.
- The atom that is bonded to the hydrogen atom has a high electronegativity, making the bond suitably polar. A strongly electronegative atom connected to the hydrogen atom must be present in the molecule. The degree of polarisation of the molecule increases with increasing electronegativity.
- The electronegative atom attached to the Hydrogen atom should be smaller in size. Greater the electrostatic attraction, the smaller the size. So, the smaller size should attract the bonding electron pair effectively.
Effects of Hydrogen Bonding on Elements
- Dissociation: HF dissociates in water and sends off the difluoride ion instead of the fluoride ion. In HF, this is due to hydrogen bonding. HCl, HBr, and HI molecules do not form hydrogen bonds. This explains why chemicals like KHCl2, KHBr2, and KHI2 don’t exist.
- Association: Because of hydrogen bonding, carboxylic acid molecules exist as dimers. Such compounds have molecular weights that are twice as large as those calculated from their simple formula.
Examples of Hydrogen Bonding
various examples of Hydrogen Bonding are,
Hydrogen Bond in Water (H2O)
An excellent example of Hydrogen Bonding is Water. A highly electronegative oxygen atom is connected to a hydrogen atom in the water molecule. The shared pair of electrons are attracted to the oxygen atoms more, and this end of the molecule becomes negative, while the hydrogen atoms become positive.

Hydrogen Bond in Water
Hydrogen Bond in Hydrogen Fluoride (HF)
A stronger-than-average hydrogen bond is created by hydrofluoric acid and is known as a symmetric hydrogen bond. Formic acid can also make this type of bond.
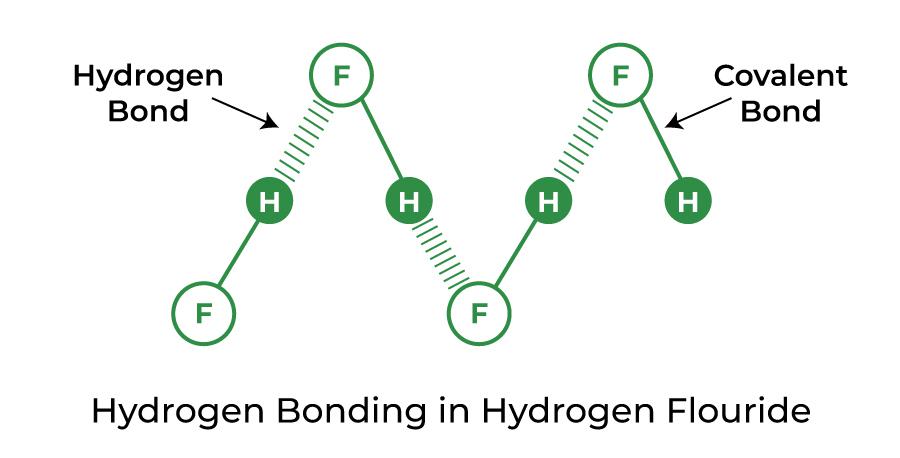
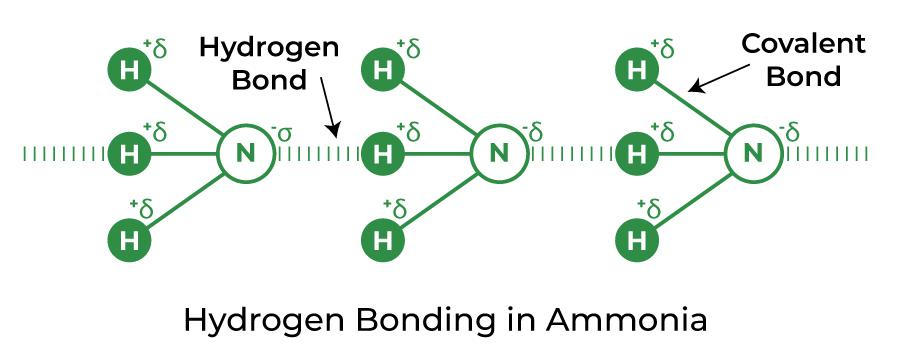
Hydrogen Bond in Ammonia (NH3)
Between the hydrogen in one molecule and the nitrogen in another, hydrogen bonds are formed. Since each nitrogen has a single electron pair, the bond that develops in the case of ammonia is relatively weak. Methylamine also has this form of hydrogen bonding with nitrogen.
Hydrogen Bond in Alcohol and Carboxylic Acid
A type of chemical molecule with a -OH group is alcohol. In most cases, hydrogen bonding is easily generated if any molecule containing the hydrogen atom is immediately coupled to either oxygen or nitrogen.
Hydrogen Bond in Alcohol
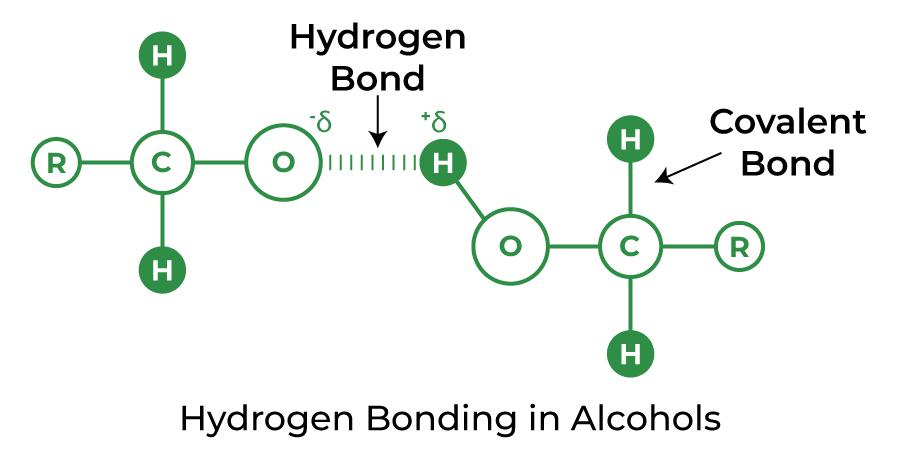
Hydrogen Bond in Alcohols
Hydrogen Bond in Carboxylic Acid is shown ion the i9mage added below,

Hydrogen Bond in Carboxylic Acid
Strength of Hydrogen Bond: The hydrogen bond is a relatively weak one. Hydrogen bonds have a strength that is halfway between weak van der Waals forces and strong covalent bonds. The attraction of the shared pair of electrons, and hence the atom’s electronegativity, determines the hydrogen bond’s dissociation energy.
Properties of Hydrogen Bonding
- Volatility – The boiling point of compounds incorporating hydrogen bonding between distinct molecules is greater, hence they are less volatile.
- Solubility – Because of the hydrogen bonding that can occur between water and the alcohol molecule, lower alcohols are soluble in water.
- Lower density of ice than water – In the case of solid ice, hydrogen bonding causes water molecules to form a cage-like structure. In fact, each water molecule is tetrahedrally connected to four other water molecules. In the solid state, the molecules are not as tightly packed as they are in the liquid state. This case-like structure collapses as ice melts, bringing the molecules closer together. As a result, the volume of water reduces while the density increases for the same quantity of water. As a result, at 273 K, ice has a lower density than water. Ice floats because of this.
- Viscosity and Surface Tension – Hydrogen bonding is found in compounds that have an associated molecule. As a result, their flow becomes more complicated. They have high surface tension and higher viscosity.
Why do hydrogen-bonded compounds have high melting and boiling points?
The melting and boiling temperatures of hydrogen-bonded compounds are unusually high. The elevated melting and boiling points of hydrogen-bonded compounds are attributable to the additional energy required to break these bonds.
- At room temperature, H2O is a liquid, whereas H2S, H2Se, and H2Te are all gases. Hydrogen bonding produces links in the water molecules, resulting in water having a higher boiling point than the other chemicals.
- Because there is hydrogen bonding in NH3, but not in PH3, ammonia has a higher boiling point than PH3.
- The presence of hydrogen bonding accounts for hydrogen fluoride’s particularly high boiling point among the halogen acids.
- Because ethanol contains hydrogen bonds, it has a higher boiling point than diethyl ether.
Types of Hydrogen Bonding
There are two types of H bonds, which are labeled as follows:
Intermolecular Hydrogen Bonding
Intermolecular hydrogen bonding occurs when hydrogen bonds are formed between molecules of the same or distinct substances. Hydrogen bonding in water, alcohol, and ammonia, for example.
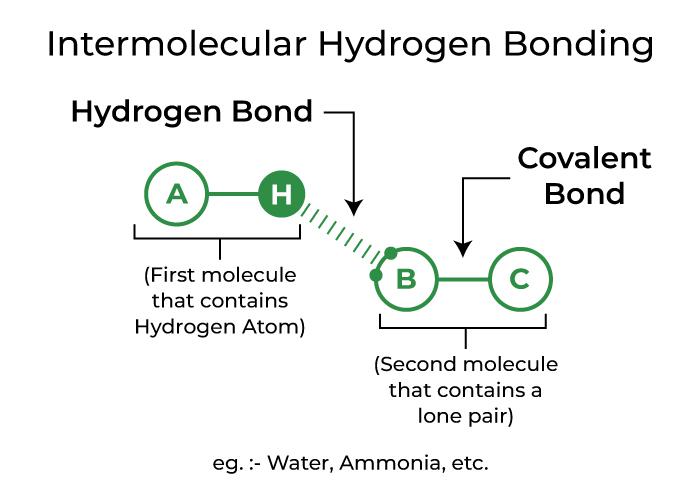
Depiction of Intermolecular Hydrogen Bonding
Intramolecular Hydrogen Bonding
Intramolecular hydrogen bonding refers to hydrogen bonding that occurs within a single molecule. It occurs in compounds with two groups, one of which has a hydrogen atom linked to an electronegative atom and the other of which has a highly electronegative atom linked to a less electronegative atom of the other group. The link is created between the more electronegative atoms of one group and the hydrogen atoms of the other group.
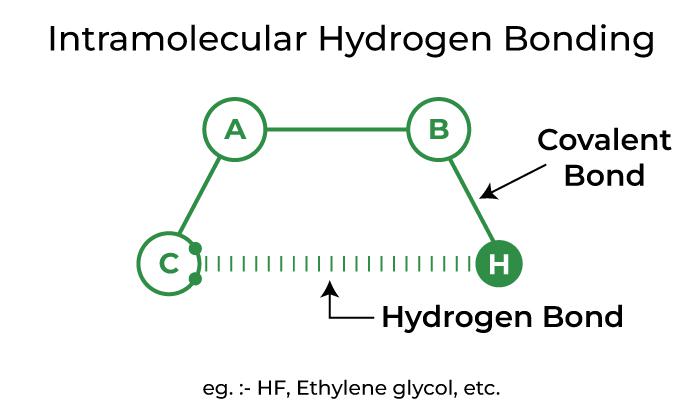
Depiction of Intramolecular Hydrogen Bonding
Difference between a Hydrogen Bond and Covalent Bond
The distinction between hydrogen bonds and covalent bonds lies primarily in their mechanism of formation, strength, and the nature of the interaction:
- Formation:
- Covalent bonds form through the sharing of electron pairs between atoms, resulting in a strong bond.
- Hydrogen bonds form due to the attraction between a hydrogen atom bonded to an electronegative atom and another electronegative atom in a different molecule or within the same molecule.
- Strength:
- Covalent bonds are relatively strong and require a substantial amount of energy to break.
- Hydrogen bonds are weaker compared to covalent bonds but stronger than van der Waals forces, which are another type of intermolecular force.
- Nature of Interaction:
- Covalent bonds involve the sharing of electrons, leading to a stable molecular structure.
- Hydrogen bonds are dipole-dipole attractions between molecules or within the same molecule, contributing to the structure and properties of substances such as water, DNA, and proteins.
Practice Questions on Hydrogen Bonds
Q1: Describe the formation and significance of hydrogen bonds in water molecules. How do hydrogen bonds contribute to the unique properties of water?
Q2: Explain the role of hydrogen bonds in maintaining the secondary structure of proteins. Provide examples of secondary structures stabilized by hydrogen bonding.
Q3: Discuss the importance of hydrogen bonds in the stability of the DNA double helix structure. How do hydrogen bonds contribute to the pairing of nitrogenous bases in DNA?
Q4: Describe the formation of hydrogen bonds between molecules of ammonia (NH3) and water (H2O). Include the polarity of molecules and the arrangement of hydrogen bonds in your explanation.
Q5: Compare and contrast hydrogen bonds with other types of intermolecular forces, such as dipole-dipole interactions and van der Waals forces. Provide examples to illustrate the differences in strength and mechanism of each type of interaction.
FAQs on Hydrogen Bonding
State Octet Rule.
Atoms are most stable when their valence shells are filled with eight electrons, according to the octet rule. It is based on the observation that the atoms of the major group elements have a proclivity for chemical bonding in such a way that each atom in the resulting molecule has eight electrons in the valence shell. Only the core group elements are subject to the octet rule.
What are the factors that affect the formation of Ionic Bond?
Factors affecting the formation of Ionic Bond are:-
- Ionization Enthalpy
- Electron Gain Enthalpy
- Lattice Energy
List any three characteristics of Ionic Compounds.
Characteristics of Ionic Compounds are as follows:
- The melting and boiling points of ionic compounds are usually quite high. This is due to the strong electrostatic forces that hold ions together in ionic compounds.
- Ionic compounds are frequently found in solid form.
- Ionic compound solutions are excellent electrical conductors. In their molten condition, they are also good conductors of electricity.
What is Hydrogen Bonding?
When a hydrogen atom is coupled to a highly electronegative atom, the shared pair of electrons are attracted more by this atom, and the molecules’ negative end becomes slightly negative while the positive end becomes slightly positive.The negative end of one molecule attracts the positive end of the other, resulting in the formation of a weak bond. This connection is referred to as Hydrogen Bonding.
What are the conditions required for Hydrogen Bonding?
Conditions required for Hydrogen Bonding are:
- A highly electronegative atom must be coupled to the hydrogen atom in the molecule. The polarisation of a molecule is proportional to its electronegativity.
- Electronegative atom should be modest in size. The greater the electrostatic attraction, the smaller the size.
Is CH4 a hydrogen bond?
No, CH4 (methane) does not exhibit hydrogen bonding. Hydrogen bonding occurs between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule or within the same molecule. In methane, hydrogen atoms are bonded to carbon, which is not electronegative enough to form hydrogen bonds.
Can HCl form hydrogen bonds?
Yes, HCl (hydrogen chloride) can participate in hydrogen bonding, but to a limited extent. Hydrogen bonding requires a hydrogen atom bonded to an electronegative atom like chlorine in HCl. However, the strength of hydrogen bonding in HCl is weaker compared to compounds like water or ammonia.
Why is ice lighter than water?
Ice is lighter than water because of its unique crystal structure. In ice, water molecules form a regular hexagonal lattice held together by hydrogen bonds, creating open spaces between the molecules. This arrangement causes ice to be less dense than liquid water, which is why ice floats on water.
Why is hydrogen bond weak?
Hydrogen bonds are relatively weak compared to covalent bonds because they involve electrostatic attractions between partially charged atoms rather than the sharing of electrons. Additionally, hydrogen bonds are weaker than covalent bonds due to their longer bond lengths and the nature of the interaction.
What is the weakest bond?
Van der Waals forces, including dispersion forces and dipole-dipole interactions, are generally considered the weakest types of chemical bonds. These forces arise from temporary fluctuations in electron distribution and are weaker than both covalent and hydrogen bonds.
What are 3 examples of a hydrogen bond?
Three examples of hydrogen bonds include:
- Hydrogen bonds between water molecules, which contribute to the unique properties of water.
- Hydrogen bonds between complementary nitrogenous bases in DNA, which stabilize the double helix structure.
- Hydrogen bonds between ammonia molecules (NH3), which play a role in ammonia’s properties as a polar molecule.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...