Difference Between Homogeneous and Heterogeneous Mixture
Last Updated :
04 Apr, 2024
Mixture is a combination of two or more substances that are not chemically bonded together. These substances can be elements, compounds, or both. There are two types of mixtures: Homogeneous and Heterogenous Mixture.
In homogeneous mixture, components are uniformly distributed and not easily distinguishable. While in heterogeneous mixture, components are not uniformly distributed and can be visually distinguished. Examples of mixtures include air, saltwater, and soil.
Let’s learn about difference between homogeneous mixtures and heterogeneous mixtures in detail.
Types of Mixtures
Mixtures are of two types.
- Homogeneous Mixture
- Heterogeneous Mixture

Difference between Homogeneous and Heterogeneous Mixtures
Below is the difference between Homogeneous and Heterogeneous Mixtures.
|
Homogeneous vs. Heterogeneous Mixtures
|
|
Homogeneous Mixture
|
Heterogeneous Mixture
|
| Mixtures that have uniform composition. |
Mixtures that do not have uniform composition throughout. |
| The boundary of separation could not be seen. |
The boundary of separation of constituent particles is clearly visible. |
| Particles are not indistinguishable. |
Particles can be physically distinguishable. |
| ‘Homo’ means the same. |
‘Hetero’ means different. |
| Particles can’t be seen with the naked eye. |
Particles can be seen with the naked eye. |
| It has uniform properties throughout. |
Its different parts have different properties. |
| For Example– Copper sulphate dissolves in water. |
For Example– Muddy water, or detergent solution. |
Homogeneous Mixture
Homogeneous Mixtures are the mixtures in which the mixed components are evenly spread in the whole mixture. There is no partitional difference in the mixture.
We can’t determine the mixture by only seeing it because its particles are not physically detectable. All Homogeneous mixtures are solutions. For example- Wine, Vinegar, etc.

Properties of Homogeneous Mixtures
Various properties of Homogeneous Mixtures are,
- It has a uniform composition throughout, for instance, you will notice that a mixture of salt and water tastes salty at each and every sip means salt has occupied its space all over uniformly.
- There is no partition in between the solution means if you will notice the saltwater you will see that there are no patches or foam or anything which will divide the solution into two halves.
Heterogeneous Mixture
Heterogenous Mixture are the mixtures in which different particles are physically distinguishable as they do not have uniform composition. These mixtures can be concluded just by watching.
In this kind of mixture, particles are physically distinguishable as they do not have uniform composition. These mixtures can be concluded just by watching.
All heterogeneous mixtures are colloid and suspended. Mixtures in which substances remain distinct and one substance disperses into another substance in the form of small bubbles or in any form are called heterogeneous mixtures. For example- Ice cubes in cola form a heterogeneous mixture. Fruit and nut cookies are a heterogeneous mixture.

Properties of Heterogeneous Mixtures
Various properties of Heterogeneous Mixtures are:
- For instance, you can take a solution of sugar and sand dissolved in water. On observing after some time, you will see that there are two halves divided in the solution in which sand has settled down in the bottom whereas sugar gets fully dissolved in the water creating the first half.
- A mixture of two immiscible liquids is also a heterogeneous mixture, like a mixture of water and oil is also a heterogeneous mixture because when they both will be mixed together, the oil will start floating on the top surface, and they will not get mixed with each other forming two clear phases in the mixture.
Examples of Homogeneous and Heterogeneous Mixtures
Air is a homogeneous mixture which is composed of various gases such as Nitrogen, Oxygen and others. Blood is a heterogeneous mixture which is made up of various components called red blood cells, white blood cells, platelets, and plasma. These substances are not properly mix together but are kept separated from each other.
Concrete consists of water, cement, gravel, sand and rocks but not in a fixed ratio. i.e. it is not always uniform and hence it is a heterogeneous mixture.
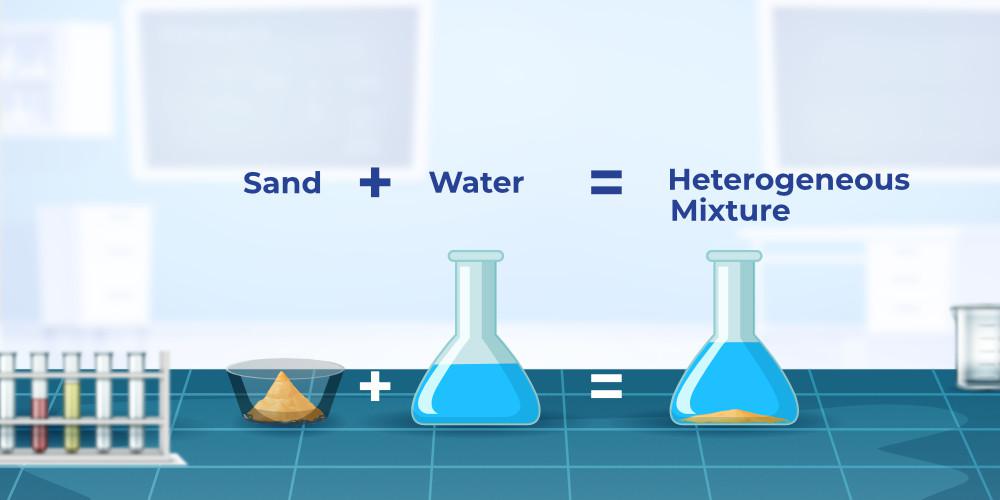
Heterogeneous Mixture Example
The image given below shows a heterogeneous mixture.
Homogeneous Mixture Example
The image given below shows a homogeneous mixture.

Must Check
Difference between Homogeneous and Heterogeneous Mixtures – FAQs
What is Homogeneous Mixture?
A Mixtures in which all the components added are spread evenly is called homogeneous mixture. An example of a homogeneous mixture is Air.
What is Heterogeneous Mixture?
A mixture in which all the components are easily distinguishable is called a Heterogeneous mixture. An example of a homogeneous mixture is Blood.
Give Heterogeneous and Homogeneous Mixture Examples?
- Various examples of homogeneous mixtures are water, lemonade, alloys, and bitumen.
- Examples of heterogeneous mixtures are sand in oil, sand in water, concrete, milk and others.
Classify matter on the basis of its composition.
On the basis of composition, matter can be classified into
- Pure substance
- Mixture
Is Air Homogeneous or Heterogeneous?
Air is a homogeneous mixture as all its components are uniformly mixed and it is difficult to separate various components separately.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...