First Order Radioactive Decay
Last Updated :
20 Sep, 2022
The phenomenon of spontaneous emission of radiation by radioactive substances came to be known as radioactivity. A naturally occurring heavy nucleus is unstable. It spontaneously emits a particle, without the stimulus of any outside agency, transforming into a different nucleus. Such a nucleus is said to be radioactive, and the process of transformation is called radioactive decay.
First Order Radioactive Decay Law
The first-order radioactive decay law states that the rate of decay (number of disintegrations per second) is proportional to the number of radioactive atoms (N) present at that time t.
N = No e-λt
where,
- N is the active nuclei after time t
- No is the initial number of nuclei
- λ is the decay constant
- t is the time
Derivation
It is known that,
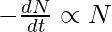
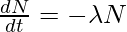 , where, λ = decay constant
, where, λ = decay constant
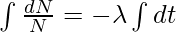
Integrating both sides we get,
logeN = -λt + c
Putting t = t0 and N = N0, the equation becomes,
logeNo = c
logeN = −λt + logeN0
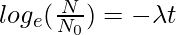
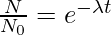
N = No e-λt
The above equation represents the radioactive decay law. It gives the number of active nuclei left after time t. Thus, the number of active nuclei in a radioactive sample decrease exponentially with time.
Decay Constant
The decay constant is the rate of decay of radioactive atoms per active atom. It is also called the disintegration constant. The radioactive constant may be defined as the reciprocal of the time interval during which the number of active nuclei in a given radioactive sample reduces to 36.8% (or 1/e times) of its initial value. It gives the probability per unit time for a nucleus of a radioactive substance to decay. The value of λ depends on the nature of the radioactive substance.
λ = 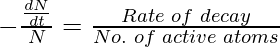
It is known that, t = 1/ λ , then we get,
N = N0 e-1
= N/2.718
= 0.368N0
So, N = 36.8% of N0
Half Life (T)
The time in which the number of radioactive atoms reduces to half of its initial value is known as half-life i.e., at t= T, N = N0/2 from the radioactive decay law.
Relation Between Half-Life and Decay Constant
In radioactive decay, after half-life, the active number of nuclei in a radioactive specimen becomes half of its initial value.
For a half life, t = T and N= N0/2.
Putting these values in equation we get,
N0/ 2 = N0 e-λT
e-λT = 1/2
e-λT = 2-1
eλT = 2
λT = loge2
T = loge2/λ
T = 0.693/λ
Average or Mean Life (τ)
The average time for which the nuclei of a radioactive sample exist is called the mean life or average life of that sample. It is equal to the ratio of the combined age of all the nuclei to the total number of nuclei present in the given sample. It is represented by τ.
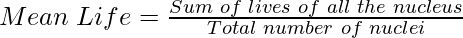
Average life is greater than the half-life i.e., τ > T. The average life of a radioactive substance is constant. It does not change with temperature and pressure.
Relation Between Mean Life and Decay Constant
Suppose a radioactive sample contains N0 nuclei at time t. After time 1 it reduces to N. Let dN nuclei disintegrate in time t to t + dt. As dt is small, so the life of each of the dN nuclei can be approximately taken equal to t.
Total life of dN nuclei = t dN
Total life of all N0 nuclei = 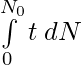
Mean Life: τ = Total life of all N0 nuclei / N0
τ = 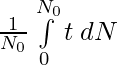
As N = N0 e-λt
dN = -λN0 e-λt dt
When N = N0, t = 0 and when N = 0, t = ∞. Changing the limits of integration in terms of time,
τ = 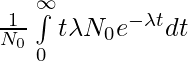
Here, we have ignored negative sign which just tells that N decreases with the passage of time t.
τ = ![Rendered by QuickLaTeX.com \lambda\int\limits_0^\infty te^{-\lambda t}dt = \lambda\bigg[\bigg(\frac{te^{-\lambda t}}{-\lambda}\bigg)_0^\infty-\int\limits_0^\infty\frac{e^{-\lambda t}}{-\lambda}dt\bigg]](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-f3abeba4874cfe1fa16dd1a5877b6d3d_l3.png)
τ = 0 + 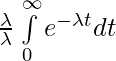
τ = ![Rendered by QuickLaTeX.com \int\limits_0^\infty e^{-\lambda t} dt = \bigg [\frac{e^{-\lambda t}}{-\lambda}\bigg ]_0^\infty](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-c7b7922ab292f465c80f4f1ad7350507_l3.png)
τ = -1/λ[e-∞ – e0] = -1/λ [0 – 1]
τ = 1/λ
Activity Of Radioactive Substance (A)
The activity of a radioactive substance is the rate of disintegration of a radioactive substance with respect to time (number of disintegration/sec). It is denoted by A.
A = – 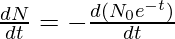
A = λ N0e-λt
A = A0 e-λt
A0 = λ N0 is the activity at time t = 0.
So, activity of a radioactive sample decreases exponentially with time.
Relation Between Activity and Half-Life
![Rendered by QuickLaTeX.com \bold{\frac{A}{A_0}= \big[\frac{1}{2}\big]^{t/T}}](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-471be2e63065ddcaca642b82beb6bebd_l3.png)
If n half-lives are used then the value becomes, N = N0/2n or A = A0/2n.
So, t= n × T.
Units of Radioactivity
- Becquerel: In S.I. system the unit of radioactivity is becquerel. 1 becquerel = 1 disintegration/sec = 1 dps.
- Rutherford: It is defined as a unit of activity equal to 104 dps.
- Curie: The traditional unit of activity is Curie. It is defined as 3.7×1010 dps which is equal to the radioactivity of 1g of pure Radium.
Solved Examples On Radioactivity
Example 1: A radioactive substance has a half-life of 4 months. In how much time the 3/4th of the substance will be decayed?
Solution:
Amount of substance decayed = 3/4
Remaining substance = N0/ 4
N0/ 2n = N0/ 4
n = 2
t = n × T
= 2×4
= 8 months
Example 2: What percentage of a given mass of a radioactive substance will be left undecayed after five half-life periods?
Solution:
Number of half-life periods (n) = 5
N/N0 = 1/2n
= 1/25
= 1/32
So, N/N0 = 3.125%
Example 3: Plutonium decays with a half-life of 2400 years. If plutonium is stored for 7200 years what fraction of its remains?
Solution:
No of half-lives (n) = Time of disintegration(t) / Half-life period (T)
n = 7200/2400 = 3
N/N0 = 1/23 = 1/8
Example 4: What is the decay constant of a radioactive substance whose half-life is 5 hours?
Solution:
λ = 0.693/T
λ = 0.693/ (5×3600)
λ = 3.85×10-5 per sec
Example 5: The half-life of a radioactive substance is 34.65 minutes. If 1022 atoms are active at any time, then find the activity of the substance.
Solution:
![Rendered by QuickLaTeX.com \frac{A}{A_0}= \big[\frac{1}{2}\big]^{t/T}](https://www.geeksforgeeks.org/wp-content/ql-cache/quicklatex.com-a87eaf14dc9368e537265a31fabc104d_l3.png)
A = -dN/dt = λN
A = (0.693× N)/T
= 0.693×1022/34.65×60
= 3.34×1018 disintegration/sec
FAQs On Radioactivity
Question 1: Write the relation between half-life and decay constant.
Answer:
The half life is evaluated by dividing 0.693 by the decay constant.
T = 0.693/λ
Question 2. Define radioactivity.
Answer:
The phenomenon of spontaneous emission of radiation by radioactive substances came to be known as radioactivity.
Question 3: Define Half-life.
Answer:
The time in which the number of radioactive atoms reduces to half of its initial value is known as half-life i.e., at t= T, N = N0/2 from the radioactive decay law.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...