The speed of light is determined by the nature of the medium through which it travels. As a result, as the light beam goes from one medium to another, it is deviated or refracted from its initial course. When a ray of white light is transmitted through a prism, it is seen that the wave with the shorter wavelength bends more than the wave with the longer wavelength.
Because typical white light is made up of waves with all of the visible wavelengths, a ray of white light is divided into a sequence of coloured bands known as the spectrum. The red light, which has the longest wavelength, deviates the least, while the violet light, which has the shortest wavelength, deviates the most. The visible spectrum of white light runs from violet at 7.50×1014 Hz to red at 4×1014 Hz. This is referred to as a continuous spectrum. Because violet blends into blue, blue into green, and so on. When a rainbow forms in the sky, it produces a similar spectrum.
Keep in mind that visible light is only a minor part of electromagnetic radiation. When electromagnetic radiation interacts with matter, atoms and molecules can absorb energy and transition to a higher energy state. These are in an unstable state because of the increased energy. Atoms and molecules release radiations in various parts of the electromagnetic spectrum to return to their normal (more stable, lower energy states) energy state.
Evidence for Quantized Electronic Energy Levels
Planck’s Contribution
In some systems, energy is quantized, which means that the system can only have particular energies and not a continuum of energies, as in the classical situation. This would be analogous to a car only being able to move at specific speeds because its kinetic energy can only have certain values. We also discover that some types of energy transfer occur with discrete chunks of energy. While most of us are aware that matter may be quantized into lumps called atoms, molecules, and the like, we are less aware that energy can also be quantized. The quantization of energy provided some of the earliest indications of the superiority of quantum mechanics over conventional physics.
Planck was able to appropriately characterise the scientifically known geometry of the blackbody spectrum via oscillator quantization. This was the first demonstration that energy may be quantized on a tiny scale, and it earned him the Nobel Prize in Physics in 1918. Despite the fact that Planck’s theory is based on observations of a macroscopic object, its analysis is based on atoms and molecules. Planck himself was hesitant to embrace his own theory that energy levels are not continuous because it was such a radical departure from traditional physics. Einstein’s explanation of the photoelectric effect, which advanced energy quantization, substantially increased general acceptance of Planck’s energy quantization.
Emission and Absorption Spectra
When electromagnetic radiations interact with matter’s atoms and molecules, the electrons in these atoms may absorb energy and leap to a higher energy state, causing them to lose stability. They must return from the higher energy level to the prior lower energy state in order to reestablish their stability. To do so, these atoms and molecules release radiations in diverse parts of the electromagnetic spectrum.
- The emission spectrum is the range of radiation released by electrons in excited atoms or molecules. An emission spectrum is defined as the spectrum of radiation released by a substance that has absorbed energy. Excited atoms, molecules, or ions are those that have absorbed radiation. To generate an emission spectrum, energy is applied to a sample by heating or irradiating it, and the wavelength (or frequency) of the radiation released as the sample releases the absorbed energy is measured.
- An absorption spectrum is analogous to an emission spectrum’s photographic negative. A radiation continuum is passed through a sample that absorbs various wavelengths of radiation. The black intervals in the dazzling continuous spectrum are caused by the missing wavelength, which corresponds to the radiation absorbed by matter.
- Spectroscopy refers to the study of emission or absorption spectra. As previously stated, the visible light spectrum was continuous, as all wavelengths (red to violet) of visible light were represented in the spectra. The emission spectra of atoms in the gas phase, on the other hand, do not show a continuous range of wavelengths from red to violet, but rather emit light at specific wavelengths with dark gaps between them.
- Because the appearance of bright lines in the spectra identifies the emitted radiation, such spectra are known as line spectra or atomic spectra. In the study of electronic structure, line emission spectra are of tremendous importance. Every element has a distinct line emission spectrum.
- In the same way that fingerprints are used to identify people, the characteristic lines in atomic spectra can be utilised in chemical analysis to identify unknown atoms. The perfect matching of lines in an emission spectrum of atoms of a known element with lines in an unknown sample swiftly proves the latter’s identity.
- Robert Bunsen (1811-1899), a German chemist, was one of the first to use line spectra to identify elements. Elements such as rubidium (Rb), caesium (Cs), thallium (Tl), indium (In), gallium (Ga), and scandium (Sc) were identified through spectroscopic analysis of their crystals. The spectroscopic approach was used to find the element helium (He) in the sun.
Line Spectrum of Hydrogen
We all know that electrons in an atom or molecule absorb energy and become excited, leap from a lower to a higher energy level, and emit radiation when they return to their original states. This phenomenon also explains the emission spectrum via hydrogen, sometimes known as the hydrogen emission spectrum.
In the late 1800s, it was discovered that when a gas is excited with an electric discharge and the light released is examined through a diffraction grating, the spectrum observed consists of discrete lines with well-defined wavelengths rather than a continuous band of light. Experiments revealed that the wavelengths of the lines matched the chemical element emitting the light. They were an atomic fingerprint created by the atom’s intrinsic structure.
The hydrogen spectrum is a crucial piece of evidence for demonstrating an atom’s quantized electronic structure. When an electric discharge is transmitted across a gaseous hydrogen molecule, the hydrogen atoms dissociate. It causes electromagnetic radiation to be emitted, which is begun by energetically excited hydrogen atoms. The hydrogen emission spectrum is made up of discrete frequency radiation. The radiation series are named after the scientists who discovered them.
When an electric discharge is conducted over gaseous hydrogen, the H2 molecules dissociate and the energetically excited hydrogen atoms created emit discrete frequency electromagnetic radiation. The hydrogen spectrum is made up of multiple series of lines that have been named after their discoverers. On the basis of actual findings, Balmer demonstrated in 1885 that if spectral lines are represented in terms of wavenumber, the visible lines of the hydrogen spectrum satisfy the following formula: where n is an integer equal to or greater than 3 (i.e., n = 3,4,5,… The Balmer series refers to the series of lines described by this formula. The only lines in the hydrogen spectrum that occur in the visible area of the electromagnetic spectrum are the Balmer series.
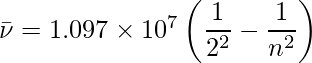
1.097 × 107 is known as the hydrogen Rydberg constant. The first five series of lines that correspond to n1 = 1, 2, 3, 4, 5 are known as the Lyman, Balmer, Paschen, Bracket, and Pfund series, respectively.
The hydrogen atom has the simplest line spectrum of any element. For heavier atoms, the line spectrum becomes increasingly complicated. There are, however, some characteristics that are shared by all line spectra, such as the fact that each element’s line spectrum is unique and that there is regularity in the line spectrum of each element.
Emission Spectrum & Absorption Spectrum
- An absorption spectrum is analogous to a negative of an emission spectrum.
- Electromagnetic radiations are bombarded on a sample that absorbs certain wavelengths of radiation to observe the absorption spectrum.
- The wavelength of radiation absorbed by matter contributes to the missing wavelength, leaving dark voids in the otherwise dazzling continuous spectrum.
- Every element has a distinct line emission spectrum. Spectroscopy is the study of the emission spectrum or absorption spectrum.
Sample Questions
Question 1: What are subatomic particles?
Answer:
Subatomic particles are the particles that make up an atom. This word often refers to protons, electrons, and neutrons.
Question 2: How do the atomic structures of isotopes vary?
Answer:
They differ in terms of the total amount of neutrons contained in the atom’s nucleus, as described by their nucleon numbers.
Question 3: What are the shortcomings of Bohr’s atomic model?
Answer:
The structure of an atom, according to this atomic model, provides poor spectrum predictions for larger atoms. It also fails to account for the Zeeman effect. It could only successfully explain the hydrogen spectrum.
Question 4: How can the total number of neutrons in an isotope’s nucleus be determined?
Answer:
The mass number of an isotope is calculated using the total number of protons and neutrons in the isotope. The atomic number describes the total number of protons in the nucleus. As a result, the number of neutrons is calculated by subtracting the atomic number from the mass number.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...