An electric current passing through a conductive solution generates a chemical reaction. This may result in the production of gas bubbles on the electrodes, metal deposits on the electrodes, changes in the color of solutions, and so on. It is known what is current and how it is produced. Anything which passes the current is known as a good conductor of electricity like copper and metal and which does not pass the current is a bad conductor of electricity like wood or plastic.
When the electrodes are submerged in water and a current is transmitted through the water, bubbles of oxygen and hydrogen emerge, as proven by William Nicholson, a British scientist. He observed that hydrogen bubbles developed at the electrode attached to the negative terminal, but oxygen bubbles developed at the electrode attached to the positive terminal.
Now let’s study how and why liquids conduct electricity in the present article.
Do Liquids Conduct Electricity?
Electricity does not conduct through all liquids. Nonetheless, certain liquids are strong conductors of electricity while others are poor conductors. Water containing dissolved salts and minerals conducts electricity well, whereas pure water conducts electricity poorly.
For concluding this we will be going to use the tester which we have created to check the conductivity of a material. First, we will check that our tester is working fine or not. Now if our tester is working we will connect the wire with the two ends of the tester and switch on the battery now we will see that bulb that is connected start glowing we can conclude that water can conduct electricity, and it is a good conductor of electricity. But there is always not possible that every liquid is a good conductor of electricity there is some liquid which is a bad conductor of electricity.
| |
Material tested
|
Conductivity
|
| 1. |
Lemon juice
|
Good conductor
|
| 2. |
Soda compounds
|
Good conductor
|
| 3. |
Vinegar
|
Bad conductor
|
| 4. |
Honey
|
Bad conductor
|
| 5. |
Mercury
|
Good Conductor
|
| 6. |
Distilled Water
|
Bad conductor
|
What happens when a current is passed through a conducting solution?
Electric current passing through a conductive fluid causes chemical reactions. As a result, gas bubbles may form on the electrodes. Metal deposits on electrodes are possible. Color changes in solutions are possible. The response would be affected by the solution and electrodes employed. When electricity is transmitted through water that has been combined with various compounds, the contents separate into negative and positive ions. An electric current flows via these ions. The greater the number of ions, the higher the conductivity of electricity.
How does water conduct electricity?
Pure water is a bad conductor of electricity. Any impurities, similar to salts, in the water, tend to conduct electricity. At the point when salts are broken down in the water, they separate into various electrically charged particles called ions. Salt, or sodium chloride (NaCl), separates into positive Na ions and negative Cl ions.
If you place a battery with a negative pole and a positive pole into the water, the negative particles will be drawn to the positive pole and the positive ions will be drawn to the negative pole in this way making a close circuit.
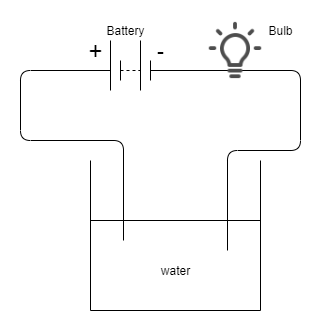
If the water is conducting then the bulb will glow, Because we know that all water is not conducting for checking the conductivity of water we dip two ends of the wire in liquid and connect to the battery if the bulb glows then water is conducting.
Electrolysis
Electrolysis is known as a process of breaking down ionic mixtures into their components by passing a direct electric current through the compound in a liquid state. The cations are reduced at the cathode and anions are oxidized at the anode.
For instance, acidified or salt-containing water can be decomposed by passing electric current to their original components hydrogen and oxygen. Liquid sodium chloride can be decomposed into sodium and chlorine particles.
During the process of electrolysis, there is an exchange of ions and atoms because of the removal or addition of electrons from the outer circuit. Essentially, on passing current, cations move to the cathode, take electrons from the cathode (given by the source battery), and are released into the neutral ion. The neutral atoms, if solid, are kept on the cathode, and if gas, moves upwards. This is a reduction process and the cation is, reduced at the cathode.
Simultaneously anions, surrender their additional electrons to the anode and are oxidized to neutral atoms at the anode. Electrons delivered by the anions traverse the electrical circuit and arrive at the cathode finishing the circuit. Electrolysis includes a synchronous oxidation response at the anode and a reduction process at the cathode.

Electrolysis
Electrolytes: An electrolyte is a mixture wherein the electrodes are mixed together. They separate on the passage of electric current. The electrodes, electrolyte and the battery together structure the electrochemical/electrolytic cell.
Electrodes: An electrode is solid electric conductors that convey electric flow into non-metallic solids, or liquids, or gases, or plasmas, or vacuums. Electrodes are ordinarily good electric conductors, however, they need not be metals. In an electrochemical cell, reduction and oxidation reactions occur at the electrodes. The electrode at which reduction takes place is known as the cathode. Oxidation happens at the anode.
Electroplating
A few times we see that a fresh new bike has sparkly handlebar and wheel edges. Be that as it may, if these are coincidentally scratched, the sparkly covering falls off uncovering a not so sparkly surface underneath. You may have additionally seen ladies utilizing jewelry, which have all are being made of gold. But, reusing several times, the gold covering wears off, uncovering silver or some other metal underneath.
We conclude that this material has the covering layer of other material on them. The process of coating desired or required material on any other substance with the help of electricity is known as electroplating.
Sample Problems
Problem 1: For what reason does a compass needle when plunged in saltwater avoid more than when dunked in drinking water?
Solution:
The seawater contains a tremendous measure of salts in comparison with drinking water, subsequently, the seawater is a good conductor of electricity, and it creates a more magnetic field in the wire and consequently diverts the compass needle more.
Problem 2: What are the factors affecting electroplating?
Solution:
Electroplating is impacted by various components. A portion of these elements includes the electrodes’ surface area, temperature, the type of metal and electrolyte utilized, and the intensity of the applied current.
Problem 3: Does pure water conduct electricity? If not, how would we be able to deal with making it conducting?
Solution:
No pure water does not conduct electricity. We can make pure water good conductor by adding salt in pure water.
Problem 4: What will you conclude about rainwater, Is it a good or bad conductor of electricity?
Solution:
Water is pure water which is a bad conductor, yet it gets blended in with air particles like Sulphur dioxide and nitrogen oxides and structures acidic solution, which is a good conductor of electricity. In this way, the compass needle shows deflection.
Problem 5: Is it ok for the electrician to complete electrical fixes outside during heavy downpours? Clarify.
Solution:
No, it isn’t ok for the electrician to complete electrical fixes outside during heavy downpours. As water comprised of broke up salts, it can conduct electricity. The electrician may get electric shock thus he should not do any electrical fixes during heavy downpours.
Problem 6: Which acid is used for electroplating?
Solution:
In the electroplating and metal completing industries, methane sulphonic acids is utilized. Methane sulphonic corrosive has progressively replaced fluoroboric acid as the picked electrolyte for the electrodeposition of tin and tin-lead solder on electronic gadgets throughout the most recent ten years.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...