Density is defined as the measurement of the weight of the object when a fixed volume of it is taken. It can be calculated by dividing the mass of an object by its mass. It is the true measure of the heaviness of the material. This can be understood with the help of the following example,
“Which weighs heavier 1 kg of cotton or 1 kg of iron?” The obvious answer to this is iron is heavier than cotton so 1 kg of iron must be heavier than 1 kg of cotton but here, both of them weigh exactly the same (1 kg) but iron feels heavier because of the density as the density of Iron is heavier.
In this article, we have provided everything related to what is density, the formula for density, density of water, and how to calculate density.
What Is Density?
The mass of a substance per unit of volume is called the density of the material. Density is explained as the tightness of the material i.e. how closely the particles are packed in the material. The tighter the material is packed the more its density.
The concept of density was first explained by the famous Greek mathematician Archimedes.
Density Definition
Density is a fundamental physical property that measures how much mass is contained within a given volume of a substance. In other words, it quantifies how tightly packed the matter in a substance is.
Density Symbol
ρ is the symbol of density. It is pronounced as Rho.
Density of Water
Density is a measure of how much mass is contained in a given volume. When we refer to the density of water, we are talking about the mass of water in a certain volume. Density is the property of the material and it can vary according to various materials. The density of water is 997 kg/m3.
Densities of Some Common Metals
The density of some common metals is discussed below in the table.
| Aluminium |
2.73 |
| Copper |
8.94 |
| Gold |
19.3 |
| Iron |
7.85 |
| Platinum |
21.4 |
| Silver |
10.5 |
| Sodium |
0.97 |
| Zinc |
7.14 |
The image for layering of different matter with different density is shown below:
.png)
The formula to calculate the density of the material is,
Density = Mass/Volume
ρ = m/V
where,
ρ is the Density of the material,
m is the mass of the material,
V is the volume of the object
Density of Various States of Matter
The density of the material is the amount of substance that is packed inside the volume of the substance. The density of the material is generally lowest in its gaseous state, greater in the liquid state, and greatest in the solid state.
The density of various states of matter is discussed in the image below,
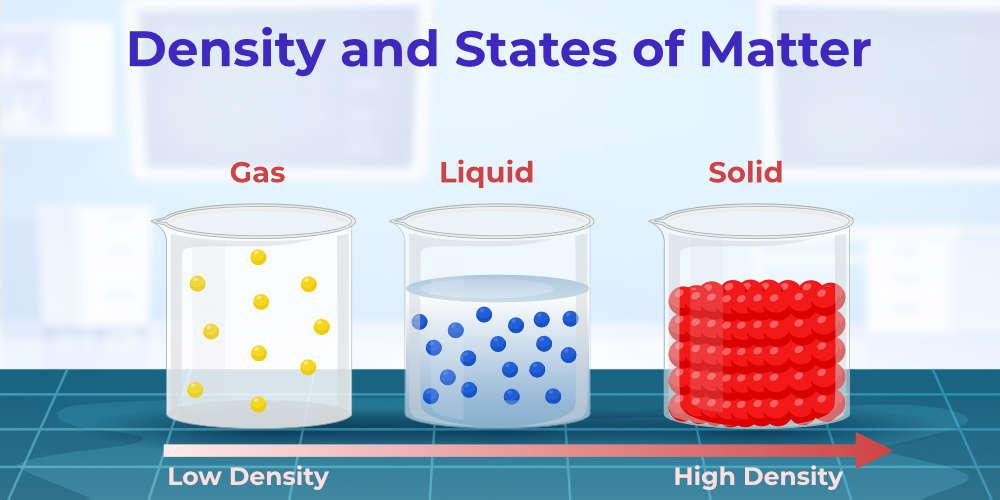
Except for the water as the density of the water is highest in the liquid state than in the solid state (ice).
Unit of Density
As density is defined as the ratio of mass and volume of any substance, mass is measured in kg and volume is measured in litre. So the density is measured in kg/m3.
Density is the property of the material and different materials have different properties, so the same volume of different materials weighs differently.
SI Unit of Density
The SI unit for measuring the density is kilogram per meter cube or kg/m3.
Other Units of Density
Other units of density which are widely used include,
- gram per centimetre cube (g/cc)
- gram per millilitre (g/mL)
- kilogram per litre (kg/L)
- kilogram per cubic decimetre (kg/dm3)
1 g/cc = 1 g/ml
Density Examples
The metals which have higher mass than other metals if the volume is kept constant are called the dense material. Platinum, Gold, etc are examples of dense metals, whereas sodium, and potassium are less dense metals.
Dense materials are materials that cannot be easily compressed whereas less dense materials such as cotton, and styrofoam are the materials that can be easily compressed.
Gaseous is one of the least dense materials as their particle are far away from each other and they can be highly compressed.
Applications of Density in Real Life
Various applications of densities are,
Separation of Substances: Various substances can be separated using density techniques. For example, oil can be separated from the water because it has a lower density than water and it floats on the surface of the water and can thus easily be removed.
Working of Submarines: Submarines go inside the water and come out of it by changing their density with respect to the water if the density of the submarine is less than the water it floats and comes out of the water. If the density of the submarine is greater than the water it goes inside the water.
Floating of Ships: The ships made of steel and other heavier metal flow despite they are much denser than water because they are shaped in such a way that their structure is always less than the water.
How Is Density Calculated?
Mathematically, the density of an object is calculated by using the formula
D = M/V
where,
D is the density of the object
M is the mass of the object
V is the volume of the object
We use the following steps to calculate the density of the given object,
Step 1: Measure and mark the mass and volume of the object given.
Step 2: Use the Density formula mass divide by the volume to calculate the density.
Step 3: Simplify the value in step 2 and unit3 to the answer obtained.
Hence, Density of the object is calculated.
Read More,
Example 1: Find the density of seawater if 1120 kg of water occupies 1m3.
Solution:
Given,
Mass of water = 1120 Kg
Volume occupied by the water = 1 m3
The density formula is,
Density = Mass/Volume
ρ = 1120/1
= 1120 kg/m3
Example 2: If a rock sample has a high carbon content and a volume of 0.055 cm3 and a mass of 0.25 g. Check whether it is Graphite or Diamond if the density of graphite is 2.266 g/cm3 and the density of diamond is 3.51g/cm3.
Solution:
Given,
Volume of rock = 0.055 cm³
Mass of the rock = 0.124 g
Density of Graphite = 2.266 g/cm3
Density of Diamond = 3.51 g/cm3
Density of Rock (ρ) = m/V
= 0.124/0.055
Density of Rock (ρ) = 2.25 g/cm3
The density is similar to graphite (2.266 g/cm3) thus the rock is Graphite.
Example 3: You’re preparing to travel to Mars. You’ve been given a 1.34-meter-long cubical box to pack. Your box’s final density must be no more than 5 kg/m3 due to fuel and space constraints. What is the maximum weight you can carry?
Solution:
Given,
A cubical box of 1.34 m in length
Volume of cubical box = 1.34 m × 1.34 m × 1.34 m
= 2.4061 m3,
Density (ρ) = 5 kg/m3
Density(ρ) = mass(m)/volume(V)
m = ρ × V
m = 5 × 2.4061
mass of the cubical box = 12.0305 kg
≅ 12 kg
Example 4: What is the density of a sugar cube that weighs 30 grams and has a side length of 8 cm?
Solution:
Given,
Mass of sugar cube = 30 g,
Volume of sugar cube = 8 cm × 8 cm × 8 cm
= 512 cm3
ρ = m/V
ρ = 30/512
ρ = 0.0585 g/cm3
FAQs on Density
1. What is Density?
Density of the material is defined as the ratio of the mass of the object with respect to its volume, i.e. the density is mass per unit volume.
2. Who discovered the principle of Density?
The principle of density was discovered by the Greek scientist Archimedes.
3. What is the formula for the Density of the material?
The formula used to calculate the density of the material is,
Density = Mass/Volume
4. What is the Density of Water?
The density of water is 997 kg/m3, or the density of water is approximately 1 gm/cc.
5. What is Bulk Density fFrmula?
Bulk density is used to calculate the density of the loose soil it is used to check if the soil is fit for agricultural purposes. The bulk density formula is,
Bulk Density = Dry Soil Weight / Volume of the Soil
6. How to find Density from Relative Density?
The relative density is the density of the material with respect to the reference material (in general the reference material is water)
Relative Density = Density of the Material / Density of Water
So to find the density of the material we multiply the relative density by the density of the water.
7. What is the Density of Water in Kg/m3 ?
Density of Water in Kg/m3 is 997.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...