If metal is reactive, its surface may be attacked slowly by air and moisture in the atmosphere. The metal reacts with the oxygen and water vapour of air forming compounds on its surface. The formation of these compounds tarnishes the metal. The compounds formed on the surface of the metal gradually falls off from the surface of the metal and then the metal underneath is attacked by air and water. This process goes on and on. In this way, the action of air and water gradually eats up the whole metal.
What is Corrosion?
The eating up of metals by the action of air, water or chemicals such as acid on their surface is called corrosion. Metals corrode when they are exposed to moist air.
For example-
Iron metal corrodes when kept in moist air for a considerable time. When an iron object is kept in moist air for a considerable time, then a red-brown substance called ‘rust’ is formed on its surface. Rust is soft and porous and it gradually falls off from the surface of the iron object and then the iron below starts corroding. So, corrosion of iron is a continuous process that ultimately eats up the whole iron object. A large amount of metal is lost every year because of corrosion. The more reactive a metal is, the faster it is to corrode it.
Corrosion of Aluminium Metal
It can be observed that the aluminium utensils lose their shine and become dull very soon. This is due to aluminium metal’s deterioration when exposed to damp air. When a gleaming aluminium metal is exposed to wet air, the oxygen in the air reacts with the metal to generate a thin, dull film of aluminium oxide all over it.
Due to the formation of this dull layer of aluminium oxide by exposure to moist air, the aluminium vessel loses its shine very soon after use. Since aluminium metal is more reactive than iron, so when in contact with moist air, fresh aluminium begins to corrode quickly.
When moist air comes into contact with aluminium metal, it forms a thin layer of aluminium oxide all over it. This aluminium oxide layer is very tough and prevents the metal underneath from further corrosion because moist air is not able to pass through the aluminium oxide layer.
So, in this way, a thin aluminium oxide layer formed on the surface of aluminium objects protect them from further corrosion.
How is corrosion of aluminium sometimes useful?
The corrosion of aluminium is sometimes useful because a newly cut piece of aluminium metal corrodes quickly to form a strong layer of aluminium oxide on its surface which then protects the aluminium piece from further corrosion. So, aluminium objects are not attacked by air and water due to the presence of protective oxide layer and hence these objects are not easily corroded. Therefore, aluminium is a common metal that is very resistant to corrosion.
Anodising
Since, on exposure of aluminium objects to moist air and water, there is a formation of a thin aluminium oxide layer on the surface of the objects, which prevents the objects from further corrosion. If this aluminium oxide layer on the surface of the aluminium objects could somehow be made thicker, then the aluminium objects would be protected from corrosion even more effectively. This can be done by a process of ‘anodising’.
The aluminium object is made an anode (positive electrode) in an electrolytic tank that electrolyzes dilute sulphuric acid. During electrolysis, oxygen gas escapes from the anode and reacts with the aluminium objects, forming a thicker layer of aluminium oxide on their surface. This thicker, more uniform aluminium oxide coating successfully shields the aluminium object from corrosion.
Thus, anodising is a process of forming a thick layer of aluminium oxide on the aluminium object by making it anode during the electrolysis of dilute sulphuric acid. The aluminium objects such as cooking utensils, pressure cookers, etc., are anodised to protect them from corrosion. The aluminium oxide layer can be dyed to give the objects some attractive colours.
Corrosion of Copper Metal
It can be observed that the copper objects gradually lose their shine due to the formation of a copper oxide layer on them. When the copper metal is exposed to the atmosphere, copper oxidizes which causes the bright copper surfaces to tarnish. After some time, the tarnish turns dark brown or black, and then green.
Copper corrosion can be classified into two types: uniform copper corrosion and non-uniform copper corrosion.
In unpolluted air, water, and non-oxidizing acids, copper corrosion occurs at insignificant rates. In oxidising acids, oxidising heavy-metal salts, sulphur, ammonia, and some sulphur and ammonia compounds, it is more sensitive to attack. Copper is toxic and can seep into both old and new homes’ tap water.
Corrosion is the cause of this leaching. Humans can tolerate a small amount of copper because it is a necessary nutrient, but too much copper can cause gastrointestinal difficulties. Copper poisoning can harm the liver and kidneys over time. When a copper object is exposed to moist air for a long period of time, copper combines with the carbon dioxide and water in the air to form a green layer of basic copper carbonate on the object’s surface. This green coating on the surface of the copper object corrodes it. This coating of basic copper carbonate is a mixture of copper carbonate and copper hydroxide 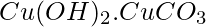 .
.
The reaction of corrosion of copper is,
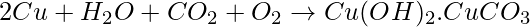
How can corrosion of copper be removed?
A green substance called basic copper carbonate is formed when copper metal corrodes in the presence of oxygen. The corrosion of copper metal is very slow because the copper metal is low in the reactivity series. A dilute acid solution can be used to clean the corroded copper vessels. The acid solution dissolves green coloured basic copper carbonate present on the corroded copper vessels and makes them look shiny and red-brown again.
Corrosion of Silver Metal
Silver is a malleable and soft grey-white metal. It’s a precious metal that’s grouped with platinum and gold, and it’s the best conductor of electricity of all metals. When silver objects are kept in the air, they lose their shine and become dull and gradually turn black. A layer of silver sulphide forms on the surface of silver when it is exposed to air.
Why is silver metal resistant to corrosion?
Silver does not react with the oxygen of air easily because silver is a highly unreactive metal. But air contains a few sulphur compounds such as hydrogen sulphide gas. As a result, the silver objects slowly react with the hydrogen sulphide gas H2S in the air, forming a black silver sulphide Ag2S coating.
The reaction of corrosion of silver is,
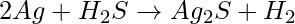
So, due to the formation of a thin silver sulphide layer on the surface of silver metals by the action of hydrogen sulphide gas present in the air, the silver metals gradually turn black. Silver metal is a shiny and bright metal that is chemically unreactive. So, the silver metal loses its shine and becomes dull very slowly. Because silver does not oxidise easily, it is known to be corrosion resistant. Therefore, silver metal is fairly resistant to corrosion.
Solved Questions
Question 1: What happens when an aluminium vessel is kept in an open atmosphere?
Answer:
Aluminium vessel gets oxidised when it is kept in open atmosphere. The oxygen in the air reacts with the vessel to generate a thin, dull film of aluminium oxide all over it.
Question 2: Give an example of a common metal that is highly resistant to corrosion.
Answer:
A common metal that is highly resistant to corrosion is aluminium.
Question 3: How does the formation of aluminium oxide on the surface of an aluminium object protect it from further corrosion?
Answer:
If the aluminium oxide layer on the surface of the aluminium objects could somehow be made thicker, then the aluminium objects would be protected from further corrosion. This can be done by a process of ‘anodising’ where a thick layer of aluminium oxide is formed on the aluminium object by making it anode during the electrolysis of dilute sulphuric acid.
Question 4: After being corroded, in which colour does the copper objects turn?
Answer:
Copper metal reacts slowly with the carbon dioxide and moisture of air to form a coating of basic copper carbonate which is green in color. So, after corrosion the color of the copper object turns into green.
Question 5: Does silver metal react with the oxygen in the air easily?
Answer:
Silver does not react with oxygen of air easily because silver is a highly unreactive metal.
Question 6: What objects can be made from silver and why?
Answer:
Silver jewellery, coins, utensils and decorative articles can be made from silver metal because of the bright shiny surface of silver and also because it is resistant to corrosion.
Question 7: Aluminium is used to make utensils for cooking even though aluminium is a highly reactive metal. Why?
Answer:
Aluminium is highly reactive metal so it corrodes quickly to form a layer of aluminium oxide on its surface. This layer is very tough and protects the object from further corrosion. So, aluminium objects are not attacked by air and water due to the presence of protective oxide layer and hence these objects are not easily corroded. Therefore, aluminium is used to make utensils for cooking even though aluminium is highly reactive metal.
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...