Ammonium Nitrate Formula – Structure, Properties, Uses, Sample Questions
Last Updated :
20 Dec, 2023
In the classification of acid, base and salt Ammonium nitrates fall under the category of inorganic salt. Which is formed by the reaction of acid like HNO3 and base like NH4OH. It is made of Nitrogen, Oxygen and Hydrogen atoms. Ammonium nitrate is used commonly in fertilisers, in pyrotechnics, herbicides, and insecticides; and in the manufacture of nitrous oxide. The study of Ammonium nitrates comes under inorganic chemistry and play a vital role in the manufacturing of many other inorganic compounds.
Based upon the current periodic table below are some comparisons between Nitrogen, Oxygen and Hydrogen:
| Properties/Element |
Nitrogen |
Oxygen |
Hydrogen |
| Chemical Symbol |
N |
O |
H |
| Atomic number |
7 |
8 |
1 |
| Atomic mass |
14 |
16 |
2 |
| Physical states |
gas |
gas |
gas |
| Valence electron |
5 |
6 |
1 |
| Valency |
3 |
2 |
1 |
| Melting point |
63.14 K |
54.36 K |
13.99 K |
| Boiling point |
77.36 K |
90.19 K |
20.28 K |
| Metal / non-metal |
non-metal |
non-metal |
non-metal |
| Percentage presence in the atmosphere |
78 |
21 |
0.00005 |
Ammonium nitrate is a colourless crystalline salt, which consists of two ions; a cation, the ammonium ion (NH4+) and anion the Nitrate ion (NO3–). The cation and anion are held together by a robust electrovalent bond. It is highly soluble in water.
- The chemical formula of Ammonium nitrate is NH4NO3. It consists of 2 nitrogen atoms, 3 oxygen atoms and 4 hydrogen atoms.
- The molecular formula of ammonium nitrate is N2H4O3.
- Molar Mass is 80.052 g/mol
Structure
Structure of ammonium nitrate is composed of two ions (Ammonium and nitrate), which can be shown as below:
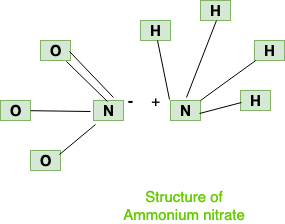
Chemical Properties
- After the explosion, this compound gives nitrogen(N2), oxygen (O2) and water.
- On heating, this compound decomposes to form nitrous oxide (N2O) and water.
- Despite being a component of the many explosives, nitrate isn’t an explosive by itself. It must be mixed with a primary explosive like an azide to make an explosive.
- When NH4NO3 is reacted with the hydroxides of alkali metals, it results in the nitrates of alkali metals and ammonia as products.
Physical Properties
- This compound has very low shock and friction sensitivities.
- The dissolution of NH4NO3 in H2O is much endothermic in nature.
- It has a trigonal crystal structure.
- Ammonium nitrate is a crystalline solid having a white or grey colour.
- It is soluble in water: having a solubility of 150g/100ml at 20°C. The solubility increase to 1024g/100ml when the temperature is raised to 100°C.
Occurrence
Laboratory formation: NH4NO3 can be prepared in laboratory from the acid base reaction between nitric acid and ammonia.
Chemical equation can be stated as follow:
NH3 + HNO3 = NH4NO3
Natural formation: Ammonium nitrate is found as the natural mineral gwihabaite in the driest regions of the Atacama Desert in Chile. It was mined there until the Haber–Bosch process made it possible to synthesise nitrates from atmospheric nitrogen, thus rendering nitrate mining obsolete.
The Haber–Bosch process, is a man-made organic process and is that the main industrial procedure for the manufacturing of ammonia today. It is named after its inventors, the German chemists Haber and Carl Bosch, who developed it within the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) employing a metal catalyst under high temperatures and pressures:
N2 + 3 H2 -> 2 NH3
Usages
Ammonium nitrate is one of the popular chemicals which is being used in different sectors like agriculture, nutrients, mining etc. Below are more detailed points for the use of ammonium nitrate.
- It is most commonly used in artificial fertilisers, in pyro techniques, herbicides and insecticides
- Used in the manufacture of nitrous oxide
- Used as an absorbent for nitrogen oxides
- Ingredient of freezing mixtures
- An oxidiser in rocket propellants
- Nutrient for yeast and antibiotic
- Widely used in explosives especially as an oil mixture for blasting rocks and in mining.
- Nitrates and nitrites are used to cure meats and to develop the characteristic flavour and pink colour to prevent rancidity and prevent the growth of clostridium botulinum spores in or on meats.
Recent Trivia: On 4th august 2020 the lebanese capital Beirut experienced a massive explosion which was caused by more than 2,700 tonnes of ammonium nitrate stored improperly.
The government has amended the rules for ammonium nitrate to curb its pilferage, introduce fire fighting provisions as well as improve ways to handle and store the chemical in the light of lessons learnt from the deadly explosion that killed about 140 people.
Sample Questions
Question 1: Is ammonium nitrate a good fertiliser?
Answer:
Ammonium nitrate fertiliser is the compound’s most common application but it also has a very volatile quality, making it useful in some industries. the use of NH4NO3 (nitrate) in gardens and large-scale growing fields stimulates plant growth and provides a ready source of nitrogen that plants can draw from.
Question 2: What happens when one mix water and ammonium nitrate?
Answer:
It feels cold when ammonium nitrate is dissolved in water which indicates this reaction is an endothermic reaction. In an endothermic reaction, the ammonium nitrate dissolves in water and the water absorbs heat rather than releases it. It contains tightly packed ionic bonds.
Question 3: List some well-known use of ammonium nitrate?
Answer:
Ammonium Nitrate is used as an ingredient for the manufacture of explosives, anesthetic gases, fertilizers, cold packs, etc.
Question 4: State the reason behind the explosive nature of ammonium nitrate?
Answer:
Ammonium Nitrate is not an explosive by its nature. However, it is one of the ingredients used for manufacturing explosives. Ammonium Nitrate is used as an oxidiser and when added with other ingredients like fuel, etc., it becomes explosive. For such explosive mixtures to explode, initiators like detonators are required.
Question 5: What are the products formed when we heat Ammonium Nitrate?
Answer:
On heating Ammonium Nitrate, Nitrous Oxide and water vapour are formed. The chemical reaction for same is given below:

Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...