In chemistry, abnormal molar masses occur when the molar masses are estimated and are higher or lower than the predicted value. The colligative qualities are used to calculate these. Elevation of boiling point, decreased relative vapour pressure, freezing point depression, and alleviation of osmotic pressure are all colligative properties. The word abnormal is in the name, implying that the way molar masses are computed, using the Van’t Hoff factor, is abnormal. Let’s have a look.
Abnormal Molar Masses
The mole masses determined by these methods do not agree with expected or theoretical values. The exact value of the molar mass can be obtained only if the following two conditions are met.
- The solutions should be diluted: The solutions used to measure the colligative properties should not be too concentrated. In concentrated solutions, the particles interact with each other as well as with the solvent. As a result, vapour pressure and, therefore, other conjugate properties depend on the nature of the solute, not just the number of solute particles.
- The solute required is not separate or collaborative in the solution: The derivative equations for measuring colligative properties are for non-electrolyte solutes that do not undergo any dissociation or have an association solution. However, discrepancies in the determination of the molar mass arise when solutes dissociate or associate with dissolution in a solvent. This is because the number of molecules in a solution changes due to the addition or dissociation of solute molecules. Therefore, abnormal molar masses are obtained as discussed below:
For substances that undergo association, dissociation, etc. in solution, the molecular mass determined by the conjugate properties differs from the expected value. This is known as abnormal molecular mass. This can be known by the Van’t half factor.
Hence, When computed from the colligative properties of solutions, the theoretical values of molecular mass are sometimes found to differ from the empirically measured values known as the Abnormal molar masses.
Association of solute particles
In some solvents, usually non-polar, solute molecules undergo bonding, that is, two, three, or even more molecules interact with each other to form larger molecules. For example, suppose that n simple molecules combine to form an associated molecule:
nA ⇆ An
(single molecules) (one molecule)
Accordingly, the total number of molecules in the solution becomes less than the number of molecules of the substance added, and, therefore, the covalent properties will be less. Since the colligative properties are inversely proportional to the molar mass of the solute, in such cases the molar mass exceeds the theoretical values. For example, in benzene solvent, both ethanoic acid (acetic acid) and benzoic acid exist as dimers:

The molar masses of dimers ethanoic acid and benzoic acid are about 120 and 244 which are almost twice their normal values of 60 and 122, respectively. The bonding of solute molecules in a solution is normal due to the hydrogen bonding between these molecules. By way of illustration, benzoic acid and ethanoic acid (acetic acid) exist as dimers due to the fabrication of hydrogen bonds.
Dissociation of solute molecules
Molecules of electrolytes (acids, bases, and salts) separate or ionize in a vent to give two or more particles. For example, AB dissociates to give a double amber of particles:
AB ⇆ A+ + B–
As a result, the total number of particles in the solution increases, and hence, the colligative properties of such solutions will be large. Since the colligative properties are inversely proportional to the molar mass, the observed molar mass will be less than the theoretical value. For example, KCI, K . set aside to give ed Cl-ions.
KCI ⇆ K+ + Cl–
This means that if we dissolve 1 mol of KCl (74.5 g) in water, we expect 1 mol of K and 1 mol of Cl– ions in the solution. So instead of 1 mole of the solution, there will be 2 moles of particles. As a result, the collateral properties will also be almost twice as high as expected. For example, if E ignores interionic attraction, 1 mol of KCl in 1 kg of water will increase the boiling point by 2 x 0.52 K (K = 0.52 Km¹) = 1.04 K. Distinctly, the molar mass of the salt should be about half of its normal value, i.e. 37.25.
Van’t Hoff Factor
Van’t Hoff factor is defined as the ratio of the normal molar mass to the observed molar mass (or abnormal molar mass) of the solute, that is, i= Normal molar mass/Observed (or abnormal) molar mass
- In the case of an association, the observed molar mass is more than the normal, the factor T has a value less than 1.
- In the case of dissociation, the Van’t Hoff factor is greater than 1 because the observed molar mass has a lower value.
- In the case of solutes that do not undergo any association or dissociation in a solvent, Van’t Hoff factor will be equal to 1 because the observed and normal molar masses will be the same.
Since the molar mass is inversely proportional to the colligative property, Van’t Hoff factor can also be defined as the ratio of the observed value of the colligative property to the ordinary value of the colligative property.
i = Observed value of the colligative property / Normal value of colligative property
or
i = Total number of moles of particles after association or dissociation / Total number of moles of particles before association or dissociation
If i > 1, solute undergoes dissociation, and if i < 1 , solute undergoes association.
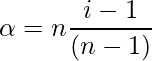
where, n = number of particles associated or dissociated.
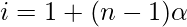
Van’t Hoff factor will always be greater than 1 for dissociation solutes. The Van’t Hoff factor is set at 1 for particles that show neither association nor dissociation. Thus, after incorporating Van’t Hoff’s factor, the colligative properties equation will be,
- Inclusion of Van’t Hoff factor modifies the equations for colligative properties as follows:
- Relative lowering of vapour pressure of solvent, (p1° -p1)/p1° = i(n2 /n1)
- Elevation in boiling point, ΔTb = iKbm
- Depression in freezing point, ΔTf = iKfm
- The osmotic pressure of the solution, π = (in2/V) RT
Sample Questions
Question 1. Why is great care taken in injecting intravenously to the same concentration as in vegetables and in a solution of blood plasma?
Answer:
During intravenous injection, the concentration of the solution for injection should be equal to that of blood plasma. If the solution is less concentrated, its osmotic pressure will be lower. Water will try to get into the red blood cells through the cell walls. As an outcome, the cells will swell and burst. On the other hand, if the solution is more concentrated, the water in the cells will try to move into the more concentrated solution outside the cell by osmosis. This causes the cells to shrink and consequently stop functioning.
Question 2. Which colligative property is privileged for characterizing the molar mass of macromolecules?
Answer:
Osmotic pressure premeditate is preferred to complete all other conjugate properties because-
- Even in dilute solutions, the osmotic pressure values are very high and can be measured accurately.
- Osmotic pressure can be premeditated at room temperature. On the other hand, the height in boiling point is measured at the higher temperature where the solute can dissociate. The depression in freezing point is measured at low temperature.
Question 3. If 0.1 mol of sugar or 0.1 mol of glucose is dissolved in one litre of water, will the depression in the freezing point be the same or different?
Answer:
The depression in the freezing point will be the same in both the solutions as both are non-electrolyte and give an equal number of solute particles.
Question 4. The outer hard shells of the two eggs are removed. One egg is placed in pure water and the other is placed in a saturated solution of sodium chloride. What will be seen and why?
Answer:
An egg placed in water will swell due to the osmosis of pure water in the egg. On the other hand, an egg placed in a saturated solution of NaCl will shrink due to the osmosis of water from the egg. This is because osmosis always occurs from a high concentration of solvent to a low concentration of solvent.
Question 5. Why is it advised to mix ethylene glycol with water in the radiator of a car while driving at a hill station?
Answer:
Ethylene glycol lowers the freezing point of water and hence, it does not freeze in a hill station.
Question 6. A solution of sodium chloride freezes at a temperature lower than water but boils at a temperature higher than water, explain.
Answer:
The freezing point of a liquid decreases upon the addition of a non-volatile solute and, therefore, a solution of sodium chloride freezes at a temperature lower than the freezing point of water. The addition of a non-volatile solute as a replacement increases the boiling point and results in the boiling point of a sodium chloride solution.
Question 6. Are equatorial solutions of sodium chloride and urea isotonic? Why?
Answer:
Sodium chloride dissociates to two ions (Na and Cl) and exerts approximately twice the osmotic pressure of 170 urea (which is non-electrolyte).
Like Article
Suggest improvement
Share your thoughts in the comments
Please Login to comment...